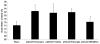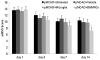Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia - PubMed (original) (raw)
Comparative Study
Comparison of the therapeutic effects of bone marrow mononuclear cells and microglia for permanent cerebral ischemia
Chao Jiang et al. Behav Brain Res. 2013.
Abstract
In this study we transplanted bone marrow mononuclear cells (BM-MNCs) or microglia into rats that had undergone permanent cerebral ischemia and observed the distribution or morphology of transplanted cells in vivo. In addition, we compared the effects of BM-MNCs and microglia on infarct volume, brain water content, and functional outcome after permanent cerebral ischemia. BM-MNCs and microglia were obtained from femur and brain, respectively, of newborn rats. Adult rats were injected with vehicle or 3 million BM-MNCs or microglia via the tail vein 24h after permanent middle cerebral artery occlusion (pMCAO). The distribution or morphologic characteristics of transplanted BM-MNCs (double stained with BrdU/Cd34 or BrdU/CD45) and microglia (double stained with BrdU/Iba-1) were detected with immunofluorescent staining at 3 or 7 and 14 days after pMCAO. Functional deficits were assessed by the modified neurologic severity score at 1, 3, 7 and 14 days after pMCAO. Brain water content was assessed at 3 days, and infarct volume was determined at 14 days. We observed more BrdU/CD45 and BrdU/Iba-1 double-stained cells than BrdU/CD34 double-stained cells around the infarcted area. Some infused microglia showed the morphology of innate microglia at 7 days after pMCAO, and the number increased at 14 days. BM-MNC-treated rats showed significantly reduced infarct volume and brain water content compared to vehicle- and microglia-treated rats. In addition, BM-MNC treatment reduced neurologic deficit scores compared to those in the other groups. The results provide evidence that infusion of BM-MNCs, but not microglia, is neuroprotective after permanent cerebral ischemia.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures
Fig. 1
Migration of microglia and BM-MNCs in vivo. Immunofluorescence staining for BrdU-labeled cells revealed that both microglia (A) and BM-MNCs (B) migrated to the boundary zone of the injured cortex in rats after infusion via the tail vein on day 3 after pMCAO. There are more CD45-positive cells (D) around the infarcted area than CD34 cells (C) (n = 4 rats per group). Scale bar = 50 μm.
Fig. 2
The number of transplanted microglia and BM-MNCs in vivo. There are more BrdU/double-stained CD45 and Iba-1 cells than BrdU/double-stained CD34 around the infarcted area at 3 days after pMCAO (A–I). Scale bar = 50 μm.
Fig. 3
The morphologic characteristics of transplanted primary microglia in vivo. The transplanted primary microglia did not show a ramified or an activated/amoeboid morphology at 3 days after pMCAO and only a few of them show a ramified or an activated/amoeboid morphology from 7 days after pMCAO. More infused primary microglia can be detected to show a ramified or an activated/amoeboid morphology (A–I). Scale bar = 50 μm.
Fig. 4
Effect of BM-MNC and microglia treatment on brain water content. Statistical analysis showed significant difference among the five groups (F = 7.37, n = 6 rats per group, P < 0.001). Post hoc analysis revealed that only BM-MNC treatment produced a significant reduction in brain water content. *P < 0.05 vs. untreated, vehicle-treated, and microglia-treated groups.
Fig. 5
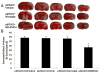
Treatment with BM-MNCs reduces infarct volume after pMCAO. (A) Representative images of TTC-stained brain slices from vehicle-treated (top), microglia-treated rats (middle) and BM-MNC-treated rats (bottom) at 14 days post-pMCAO. (B) Quantification shows that the infarct volume of BM-MNC-treated rats was significantly smaller than that of untreated, vehicle-treated, and microglia-treated rats (F = 30.80, n = 6 rats per group, P < 0.001). *P < 0.05 vs. any other group.
Fig. 6
Treatment with BM-MNCs reduces neurologic deficit after pMCAO. ANOVA revealed a significant difference in neurologic function among untreated, vehicle-treated, microglia-treated, and BM-MNC-treated groups on days 1, 3, 7 and 14 after pMCAO (F value for tests of between-subject effect = 9.33, P < 0.001). Treatment with BM-MNCs significantly improved neurologic function on day 14 compared to that in untreated, vehicle-treated, and microglia-treated groups (n = 10 rats per group). *P < 0.05 vs. all other groups.
Similar articles
- Migration towards SDF-1 selects angiogenin-expressing bone marrow monocytes endowed with cardiac reparative activity in patients with previous myocardial infarction.
Ascione R, Rowlinson J, Avolio E, Katare R, Meloni M, Spencer HL, Mangialardi G, Norris C, Kränkel N, Spinetti G, Emanueli C, Madeddu P. Ascione R, et al. Stem Cell Res Ther. 2015 Apr 11;6(1):53. doi: 10.1186/s13287-015-0028-y. Stem Cell Res Ther. 2015. PMID: 25889213 Free PMC article. - Comparsion between Intravenous Delivered Human Fetal Bone Marrow Mesenchymal Stromal Cells and Mononuclear Cells in the Treatment of Rat Cerebral Infarct.
Huang AH, Zhang PP, Zhang B, Ma BQ, Guan YQ, Zhou YD. Huang AH, et al. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016 Oct 10;38(5):497-506. doi: 10.3881/j.issn.1000-503X.2016.05.002. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016. PMID: 27825404 - Bone marrow-derived mononuclear cells ameliorate neurological function in chronic cerebral infarction model mice via improvement of cerebral blood flow.
Kitamura T, Terashima T, Katagi M, Ohashi N, Nozaki K, Tsuji A. Kitamura T, et al. Cytotherapy. 2023 Nov;25(11):1186-1199. doi: 10.1016/j.jcyt.2023.07.003. Epub 2023 Aug 8. Cytotherapy. 2023. PMID: 37552144 - Infiltration of invariant natural killer T cells occur and accelerate brain infarction in permanent ischemic stroke in mice.
Wang ZK, Xue L, Wang T, Wang XJ, Su ZQ. Wang ZK, et al. Neurosci Lett. 2016 Oct 28;633:62-68. doi: 10.1016/j.neulet.2016.09.010. Epub 2016 Sep 13. Neurosci Lett. 2016. PMID: 27637387 - Bone marrow mesenchymal stem cell transplantation downregulates plasma level and the microglia expression of transforming growth factor β1 in the acute phase of cerebral cortex ischemia.
Liang ZH, Gu JJ, Yu WX, Guan YQ, Khater M, Li XB. Liang ZH, et al. Chronic Dis Transl Med. 2020 Jul 1;6(4):270-280. doi: 10.1016/j.cdtm.2020.05.005. eCollection 2020 Dec. Chronic Dis Transl Med. 2020. PMID: 33336172 Free PMC article.
Cited by
- Cell Therapies under Clinical Trials and Polarized Cell Therapies in Pre-Clinical Studies to Treat Ischemic Stroke and Neurological Diseases: A Literature Review.
Hatakeyama M, Ninomiya I, Otsu Y, Omae K, Kimura Y, Onodera O, Fukushima M, Shimohata T, Kanazawa M. Hatakeyama M, et al. Int J Mol Sci. 2020 Aug 27;21(17):6194. doi: 10.3390/ijms21176194. Int J Mol Sci. 2020. PMID: 32867222 Free PMC article. Review. - Safety and efficacy of bone marrow mononuclear cell therapy for ischemic stroke recovery: a systematic review and meta-analysis of randomized controlled trials.
Tang Y, Wang Z, Teng H, Ni H, Chen H, Lu J, Chen Z, Wang Z. Tang Y, et al. Neurol Sci. 2024 May;45(5):1885-1896. doi: 10.1007/s10072-023-07274-x. Epub 2024 Jan 3. Neurol Sci. 2024. PMID: 38172413 Free PMC article. Review. - Premorbid Use of Beta-Blockers or Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers in Patients with Acute Ischemic Stroke.
Zeng Y, Nie K, Wallace KL, Li F, Zhang J, Li C, Wang Y, Zhang J, Wang J, Jiang C. Zeng Y, et al. Oxid Med Cell Longev. 2023 Feb 1;2023:7733857. doi: 10.1155/2023/7733857. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36778208 Free PMC article. - Bone marrow mononuclear cell transplantation promotes therapeutic angiogenesis via upregulation of the VEGF-VEGFR2 signaling pathway in a rat model of vascular dementia.
Wang J, Fu X, Jiang C, Yu L, Wang M, Han W, Liu L, Wang J. Wang J, et al. Behav Brain Res. 2014 May 15;265:171-80. doi: 10.1016/j.bbr.2014.02.033. Epub 2014 Feb 28. Behav Brain Res. 2014. PMID: 24589546 Free PMC article. - The effect of autologous bone marrow mononuclear cell transplantation on the survival duration in Amyotrophic Lateral Sclerosis - a retrospective controlled study.
Sharma AK, Sane HM, Paranjape AA, Gokulchandran N, Nagrajan A, D'sa M, Badhe PB. Sharma AK, et al. Am J Stem Cells. 2015 Mar 15;4(1):50-65. eCollection 2015. Am J Stem Cells. 2015. PMID: 25973331 Free PMC article.
References
- Jablonska A, Lukomska B. Stroke induced brain changes: implications for stem cell transplantation. Acta Neurobiologiae Experimentalis (Warsz) 2011;71:74–85. - PubMed
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circulation Research. 2003;92:692–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K01AG031926/AG/NIA NIH HHS/United States
- R01AT007317/AT/NCCIH NIH HHS/United States
- R01NS078026/NS/NINDS NIH HHS/United States
- R01 AT007317/AT/NCCIH NIH HHS/United States
- R01 NS078026/NS/NINDS NIH HHS/United States
- K01 AG031926/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous