Macrophages are required for adult salamander limb regeneration - PubMed (original) (raw)
Macrophages are required for adult salamander limb regeneration
James W Godwin et al. Proc Natl Acad Sci U S A. 2013.
Abstract
The failure to replace damaged body parts in adult mammals results from a muted growth response and fibrotic scarring. Although infiltrating immune cells play a major role in determining the variable outcome of mammalian wound repair, little is known about the modulation of immune cell signaling in efficiently regenerating species such as the salamander, which can regrow complete body structures as adults. Here we present a comprehensive analysis of immune signaling during limb regeneration in axolotl, an aquatic salamander, and reveal a temporally defined requirement for macrophage infiltration in the regenerative process. Although many features of mammalian cytokine/chemokine signaling are retained in the axolotl, they are more dynamically deployed, with simultaneous induction of inflammatory and anti-inflammatory markers within the first 24 h after limb amputation. Systemic macrophage depletion during this period resulted in wound closure but permanent failure of limb regeneration, associated with extensive fibrosis and disregulation of extracellular matrix component gene expression. Full limb regenerative capacity of failed stumps was restored by reamputation once endogenous macrophage populations had been replenished. Promotion of a regeneration-permissive environment by identification of macrophage-derived therapeutic molecules may therefore aid in the regeneration of damaged body parts in adult mammals.
Keywords: developmental biology; immunology; limb development; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
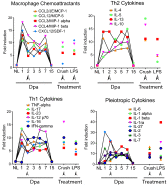
Cytokine regulation during the time course of regeneration. A cross-species cytokine protein array detects changes in cytokine/chemokine profiles in axolotl blastema at various time points after amputation relative to baseline expression in uninjured normal limb. Crush injury and LPS treatments were analyzed at 2 dpa after injury, relative to untreated limb. Arrowheads indicate 2 dpa time points. Each data point shows the mean from two separate experiments using pooled samples from six animals per time point/treatment. dpa, days postamputation; NL, normal limb.
Fig. 2.
Myeloid cells accumulate in the regenerating limb blastema. (A) α-Naphthyl acetate (NSE) enzyme cytochemistry was used to detect myeloid cells at different time points after amputation. Cell numbers were determined by counting NSE-positive cells to detect myeloid cells (B), CA esterase cytochemistry to detect granulocytes (C), and immunocytochemistry to detect monocytes/macrophages (D), using Fiji image analysis software on at least three separate experiments. Cell numbers are expressed as the number of cells counted per unit area as the mean ± SEM. Representative sections are shown in
Figs. S2
and
S3
. (Scale bars, 100 μm.)
Fig. 3.
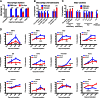
Macrophage depletion alters cytokine and chemokine induction after amputation and modulates the gene expression profiles of key regenerative genes. (A) Flow cytometric measurement of rhodamine-labeled macrophage populations in macrophage depleted (Clo-Lipo) vs. control (PBS-Lipo) animals. (B and C) Specific cytokine and chemokine profiles 4 dpa with and without macrophage depletion. Histograms show relative levels and were normalized to uninjured normal limb from the same animal. (D) Changes in gene expression profiles in the resected stump on macrophage ablation were assessed by quantitative RT-PCR, over the first 6 dpa. Plots show mean ± SEM of at least three independent experiments. *P ≤ 0.05; **P ≤ 0.01; **P ≤ 0.001, ****P ≤ 0.0001. n > 3. Primer sequences used in RT-PCR gene expression analysis are listed in
Table S1
.
Fig. 4.
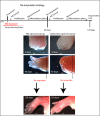
Blockade of limb regeneration depends on early macrophage depletion. (A) Macrophage depletion strategy: early phase depletion targets blastemal formation and late phase depletion targets the redevelopment phase. (B) Injection of PBS liposomes (PBS-Lipo) has no effect on normal limb regeneration (n = 10). Maximal macrophage ablation before amputation (early phase depletion) blocks limb regeneration in all animals (n = 10). Amputation plane is shown by a dotted line. Ablation of macrophages after blastemal formation (late phase depletion) allows regeneration to proceed to completion with a reproducible delay in defined regeneration stages (n = 5). (C) Control PBS-Lipo–injected animals enter paddle stage at 30 dpa and show weak collagen reactivity to trichrome staining (C′: dashed box marks inset showing high-magnification images). (D) Triple Clo-Lipo–treated animals do not initiate regeneration at 30 dpa and the failed regenerate (D′: high-magnification inset) features a complete basement membrane (arrow), a dense stratum compactum (double arrows), pronounced epidermal tongues (arrowhead), and extensive collagen deposition (asterisks). Extensive collagen deposition is still evident in Clo-Lipo stumps at 90 dpa (F and F′) relative to control PBS-Lipo regenerates (E and E′). (G–J) Picosirius red staining was used to analyze collagen fiber density. (I, I′, J, and J′) Macrophage depleted failed regenerates show dense thick collagen fiber deposition at 30 and 90 dpa compared with regenerating control limbs (G, G′, H, and H′). Thin fibers stain green and thicker fibers stain yellow/red under polarized light. (Scale bars, 100 μm.)
Fig. 5.
Extracellular matrix components are altered by macrophage depletion. At 20 dpa, Clod-Lipo–treated animals show increased (D and D′) collagen I and (E and E′) collagen IV deposition under the wound epithelium (WE) and (F and F′) an increased number of cells (marked with white arrows) staining positive for the myofibroblast marker α-SMA relative to control animals (A–C). Tiled confocal images taken at ×20 magnification. (Scale bars, 100 μm.)
Fig. 6.
Reamputation of limbs stumps after clodronate-loaded liposome treatment and macrophage repopulation reactivates a functional regeneration program. After macrophages are replenished, limb regeneration is activated by reamputation at a more proximal position that includes transection of functional nerve (n = 6). WE, wound epithelium.
Similar articles
- Midkine is a dual regulator of wound epidermis development and inflammation during the initiation of limb regeneration.
Tsai SL, Baselga-Garriga C, Melton DA. Tsai SL, et al. Elife. 2020 Jan 14;9:e50765. doi: 10.7554/eLife.50765. Elife. 2020. PMID: 31934849 Free PMC article. - Activation of germline-specific genes is required for limb regeneration in the Mexican axolotl.
Zhu W, Pao GM, Satoh A, Cummings G, Monaghan JR, Harkins TT, Bryant SV, Randal Voss S, Gardiner DM, Hunter T. Zhu W, et al. Dev Biol. 2012 Oct 1;370(1):42-51. doi: 10.1016/j.ydbio.2012.07.021. Epub 2012 Jul 27. Dev Biol. 2012. PMID: 22841627 Free PMC article. - An integrative framework for salamander and mouse limb regeneration.
Payzin-Dogru D, Whited JL. Payzin-Dogru D, et al. Int J Dev Biol. 2018;62(6-7-8):393-402. doi: 10.1387/ijdb.180002jw. Int J Dev Biol. 2018. PMID: 29943379 Review. - Neural control of growth and size in the axolotl limb regenerate.
Wells KM, Kelley K, Baumel M, Vieira WA, McCusker CD. Wells KM, et al. Elife. 2021 Nov 15;10:e68584. doi: 10.7554/eLife.68584. Elife. 2021. PMID: 34779399 Free PMC article. - Advances in Decoding Axolotl Limb Regeneration.
Haas BJ, Whited JL. Haas BJ, et al. Trends Genet. 2017 Aug;33(8):553-565. doi: 10.1016/j.tig.2017.05.006. Epub 2017 Jun 22. Trends Genet. 2017. PMID: 28648452 Free PMC article. Review.
Cited by
- The Scaffold Immune Microenvironment: Biomaterial-Mediated Immune Polarization in Traumatic and Nontraumatic Applications
Sadtler K, Allen BW, Estrellas K, Housseau F, Pardoll DM, Elisseeff JH. Sadtler K, et al. Tissue Eng Part A. 2017 Oct;23(19-20):1044-1053. doi: 10.1089/ten.TEA.2016.0304. Epub 2016 Nov 9. Tissue Eng Part A. 2017. PMID: 27736323 Free PMC article. - Cortisol-treated zebrafish embryos develop into pro-inflammatory adults with aberrant immune gene regulation.
Hartig EI, Zhu S, King BL, Coffman JA. Hartig EI, et al. Biol Open. 2016 Aug 15;5(8):1134-41. doi: 10.1242/bio.020065. Biol Open. 2016. PMID: 27444789 Free PMC article. - A PI3Kγ signal regulates macrophage recruitment to injured tissue for regenerative cell survival.
Zhou S, Liu Z, Kawakami A. Zhou S, et al. Dev Growth Differ. 2022 Oct;64(8):433-445. doi: 10.1111/dgd.12809. Epub 2022 Sep 21. Dev Growth Differ. 2022. PMID: 36101496 Free PMC article. - Immunity in salamander regeneration: Where are we standing and where are we headed?
Bolaños-Castro LA, Walters HE, García Vázquez RO, Yun MH. Bolaños-Castro LA, et al. Dev Dyn. 2021 Jun;250(6):753-767. doi: 10.1002/dvdy.251. Epub 2020 Sep 21. Dev Dyn. 2021. PMID: 32924213 Free PMC article. Review.
References
- Carlson BM. Principles of Regenerative Biology. New York: Elsevier/Academic Press; 2007. p. xix.
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310(5756):1919–1923. - PubMed
- Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3(8):566–574. - PubMed
- Parish CL, Beljajeva A, Arenas E, Simon A. Midbrain dopaminergic neurogenesis and behavioural recovery in a salamander lesion-induced regeneration model. Development. 2007;134(15):2881–2887. - PubMed
- Ferretti P, Zhang F, O’Neill P. Changes in spinal cord regenerative ability through phylogenesis and development: Lessons to be learnt. Dev Dyn. 2003;226(2):245–256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources