Angiotensin II increased neuronal stem cell proliferation: role of AT2R - PubMed (original) (raw)
Angiotensin II increased neuronal stem cell proliferation: role of AT2R
Jie Chao et al. PLoS One. 2013.
Abstract
Angiotensin II (Ang II), known a potent vasoactive substance in the renin-angiotensin system in the brain, plays a critical role in systemic blood pressure control. However, increasing evidence indicated that the physiological role of Ang II go beyond its vasoactive effect. In the present study, we demonstrated that Ang II type-1 receptor (AT1R) and type-2 receptor (AT2R) were expressed in primary rat hippocampal neuronal stem cells (NSCs). Treatment of rat hippocampal NSCs with Ang II increased cell proliferation. Pretreatment of NSCs with specific AT2R, but not AT1R, antagonist significantly suppressed Ang II-induced cell proliferation. Furthermore, Ang II stimulated ERK and Akt phosphorylation in NSCs. Pretreatment of MEK inhibitor, but not PI3K inhibitor, inhibited Ang II-induced ERK phosphorylation as well as cell proliferation. In addition, stimulation of NSCs with Ang II decreased expression of KV 1.2/KV 3.1 channels and blocked K(+) currents which lie downstream of ERK activation. Taken together, these findings underpin the role of AT2R as a novel target that regulates cell proliferation mediated by Ang II with implications for therapeutic intervention for regulation of neurogenesis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
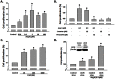
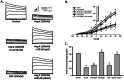
Figure 1. Effect of Ang II on NSC proliferation.
A) Ang II (0.01, 0.1, 1, 10 and 100 µM) increased NSC proliferation in a concentration-dependent manner. NSCs were treated with different concentrations of Ang II for 48 hours followed by CyQUANT assay. B) The blockade of AT1R and AT2R on NSCs proliferation. Cells were pretreated with specific antagonist to AT1R or AT2R for 1 hour followed by treatment with Ang II for another 48 hours. Cell proliferation was assessed by CyQUANT assay. C) CGP42112A (10, 100 and 1000 nM) induced increase of NSC proliferation in a concentration-dependent manner. NSCs were treated with different concentrations of CGP42112A for 48 hours followed by CyQuant assay. D) Effect of AT2R overexpression on NSC proliferation. AT2R expression in NSCs transduced with control virus and AT2R virus for 72 hours as shown in the inset. *P<0.05 vs control, #P<0.05 vs Ang II-treated group, n = 5 in each group.
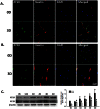
Figure 2. Expression pattern of AT1R and AT2R in NSCs.
A) Representative immunofluorescence images showing the AT1R expression in NSCs. 0D: 0 day; 3D: 3 days. NSCs cultured at day 0 or day 3 were fixed with 4% paraformaldehyde followed by immunostaining for nestin (red), a neural stem cell marker, and AT1R (green). DAPI (blue): nuclei marker. Scale bar:40 µm. B) Representative immunofluorescence images showing the AT2R expression in NSCs. NSCs cultured at day 0 or day 3 were fixed with 4% paraformaldehyde followed by immunostaining for nestin (red) and AT2R (green). DAPI (blue): nuclei marker. Scale bar:40 µm. C) Representative blots (left panel) and mean data of relative blot density (right panel) showing expression pattern of AT1R and AT2R in NSCs. Expression of AT1R or AT2R was determined from NSCs cultured for 0 hour, 12 hours, 1 day, 2 days, 3 days and 4 days by Western Blot analysis. *P<0.05 vs AT1R expression in NSCs cultured at 0 hour; #P<0.05 vs AT2R expression in NSCs cultured at 0 hour, n = 3 in each group.
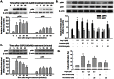
Figure 3. Ang II -induced ERK and Akt activation.
A) Representative blots (upper panel) and mean data of relative blot density (lower panel) showing Ang II induced a rapid phosphorylation of ERK and Akt in NSCs. Cells were treated with Ang II for different time followed by western- blot analysis. *P<0.05 vs untreated group; n = 3 in each group. B) Representative blots (upper panel) and mean data of blot density (lower panel) showing blockade of AT1R and AT2R on phosphorylation of ERK and Akt. NSCs were pre-treated with specific antagonist to AT1R or AT2R for 1 hour followed by incubation with Ang II for 30 min. *P<0.05 vs untreated group, #P<0.05 vs Ang II-treated group; n = 3 in each group. C) Representative blots (upper panel) and mean data of relative blot density (lower panel) showing CGP42112A induced a rapid phosphorylation of ERK and Akt in NSCs. Cells were treated with CGP42112A for different time followed by western-blot analysis. *P<0.05 vs untreated group; n = 3 in each group. D) Effect of blockade of ERK and Akt on Ang II-induced NSC proliferation. Cells were pretreated with specific ERK or Akt pathway inhibitor-U0126 or LY2940002 for 1 hour followed by treatment with Ang II for another 48 hours. Cell proliferation was assessed by CyQUANT Assay. *P<0.05 vs untreated group, #P<0.05 vs Ang II- treated group; n = 5 in each group.
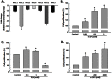
Figure 4. Ang II decreased the expression of Kv channel in NSCs.
A) Effect of Ang II on the expression of different KV channels by real-time RT-PCR assay. NSCs were treated with Ang II (0.1 µM) for 24 hours followed by RT-PCR. n = 3 in each group. B) KV channel blockade with 4-AP induced NSC proliferation in concentration-dependent manner. NSCs were treated with different concentrations of 4-AP for 48 hours followed by CyQUANT assay. C) Effect of TEA on NSCs proliferation. NSCs were treated with different concentrations of TEA for 48 hours followed by CyQUANT assay. D) α-DTX induced NSC proliferation in concentration-dependent manner. NSCs were treated with different concentrations of α-DTX for 48 hours followed by CyQUANT assay. *P<0.05 vs untreated control group, n = 5 in each group.
Figure 5. Ang II-mediated blockage of K+ currents lies downstream of AT2R/ERK pathway.
A) Representative K+ current traces showing response of Ang II, Ang II+ PD123319, Ang II+Losartan, CGP42112A or Ang II+U0126 in NSCs evoked from −80 to 80 mV. NSCs were pre-treated with vehicle, Losartan, PD123319, or U0126 for 1 hour followed by incubation with Ang II or CGP42112A for 24 hours. B) Mean data of peak K+ current change from −80 to 80 mV. C) Peak K+ current change at +80 mV. *P<0.05 vs untreated control group, #P<0.05 vs Ang II- treated group; n = 10 in each group.
Similar articles
- Dissociation of angiotensin II-stimulated activation of mitogen-activated protein kinase kinase from vascular contraction.
Watts SW, Florian JA, Monroe KM. Watts SW, et al. J Pharmacol Exp Ther. 1998 Sep;286(3):1431-8. J Pharmacol Exp Ther. 1998. PMID: 9732408 - Angiotensin II/angiotensin II type I receptor (AT1R) signaling promotes MCF-7 breast cancer cells survival via PI3-kinase/Akt pathway.
Zhao Y, Chen X, Cai L, Yang Y, Sui G, Fu S. Zhao Y, et al. J Cell Physiol. 2010 Oct;225(1):168-73. doi: 10.1002/jcp.22209. J Cell Physiol. 2010. PMID: 20458733 - Angiotensin type 2 receptor is expressed in the adult rat kidney and promotes cellular proliferation and apoptosis.
Cao Z, Kelly DJ, Cox A, Casley D, Forbes JM, Martinello P, Dean R, Gilbert RE, Cooper ME. Cao Z, et al. Kidney Int. 2000 Dec;58(6):2437-51. doi: 10.1046/j.1523-1755.2000.00427.x. Kidney Int. 2000. PMID: 11115077 - AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade.
Widdop RE, Matrougui K, Levy BI, Henrion D. Widdop RE, et al. Hypertension. 2002 Oct;40(4):516-20. doi: 10.1161/01.hyp.0000033224.99806.8a. Hypertension. 2002. PMID: 12364356 - Counteraction between angiotensin II and angiotensin-(1-7) via activating angiotensin type I and Mas receptor on rat renal mesangial cells.
Xue H, Zhou L, Yuan P, Wang Z, Ni J, Yao T, Wang J, Huang Y, Yu C, Lu L. Xue H, et al. Regul Pept. 2012 Aug 20;177(1-3):12-20. doi: 10.1016/j.regpep.2012.04.002. Epub 2012 May 1. Regul Pept. 2012. PMID: 22561449
Cited by
- Consequences of cancer treatments on adult hippocampal neurogenesis: implications for cognitive function and depressive symptoms.
Pereira Dias G, Hollywood R, Bevilaqua MC, da Luz AC, Hindges R, Nardi AE, Thuret S. Pereira Dias G, et al. Neuro Oncol. 2014 Apr;16(4):476-92. doi: 10.1093/neuonc/not321. Epub 2014 Jan 26. Neuro Oncol. 2014. PMID: 24470543 Free PMC article. Review. - Neurite outgrowth induced by stimulation of angiotensin II AT2 receptors in SH-SY5Y neuroblastoma cells involves c-Src activation.
Blanco HM, Perez CN, Banchio C, Alvarez SE, Ciuffo GM. Blanco HM, et al. Heliyon. 2023 Apr 21;9(5):e15656. doi: 10.1016/j.heliyon.2023.e15656. eCollection 2023 May. Heliyon. 2023. PMID: 37144208 Free PMC article. - Interaction between Angiotensin Type 1, Type 2, and Mas Receptors to Regulate Adult Neurogenesis in the Brain Ventricular-Subventricular Zone.
Garcia-Garrote M, Perez-Villalba A, Garrido-Gil P, Belenguer G, Parga JA, Perez-Sanchez F, Labandeira-Garcia JL, Fariñas I, Rodriguez-Pallares J. Garcia-Garrote M, et al. Cells. 2019 Nov 30;8(12):1551. doi: 10.3390/cells8121551. Cells. 2019. PMID: 31801296 Free PMC article. - Four main therapeutic keys for Parkinson's disease: A mini review.
Hernandez-Baltazar D, Nadella R, Mireya Zavala-Flores L, Rosas-Jarquin CJ, Rovirosa-Hernandez MJ, Villanueva-Olivo A. Hernandez-Baltazar D, et al. Iran J Basic Med Sci. 2019 Jul;22(7):716-721. doi: 10.22038/ijbms.2019.33659.8025. Iran J Basic Med Sci. 2019. PMID: 32373291 Free PMC article. Review. - Potential effects of commonly applied drugs on neural stem cell proliferation and viability: A hypothesis-generating systematic review and meta-analysis.
Mortimer KRH, Vernon-Browne H, Zille M, Didwischus N, Boltze J. Mortimer KRH, et al. Front Mol Neurosci. 2022 Oct 5;15:975697. doi: 10.3389/fnmol.2022.975697. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36277493 Free PMC article.
References
- de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacological reviews 52: 415–472. - PubMed
- Bumpus FM, Catt KJ, Chiu AT, DeGasparo M, Goodfriend T, et al. (1991) Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension 17: 720–721. - PubMed
- Mogi M, Horiuchi M (2013) Effect of angiotensin II type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatrics & gerontology international 13: 13–18. - PubMed
- Horiuchi M, Mogi M, Iwai M (2010) The angiotensin II type 2 receptor in the brain. Journal of the renin-angiotensin-aldosterone system : JRAAS 11: 1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous