Complete protein characterization using top-down mass spectrometry and ultraviolet photodissociation - PubMed (original) (raw)
. 2013 Aug 28;135(34):12646-51.
doi: 10.1021/ja4029654. Epub 2013 Jun 4.
Affiliations
- PMID: 23697802
- PMCID: PMC3757099
- DOI: 10.1021/ja4029654
Complete protein characterization using top-down mass spectrometry and ultraviolet photodissociation
Jared B Shaw et al. J Am Chem Soc. 2013.
Abstract
The top-down approach to proteomics offers compelling advantages due to the potential to provide complete characterization of protein sequence and post-translational modifications. Here we describe the implementation of 193 nm ultraviolet photodissociation (UVPD) in an Orbitrap mass spectrometer for characterization of intact proteins. Near-complete fragmentation of proteins up to 29 kDa is achieved with UVPD including the unambiguous localization of a single residue mutation and several protein modifications on Pin1 (Q13526), a protein implicated in the development of Alzheimer's disease and in cancer pathogenesis. The 5 ns, high-energy activation afforded by UVPD exhibits far less precursor ion-charge state dependence than conventional collision- and electron-based dissociation methods.
Figures
Figure 1
UVPD spectra of the 11+ charge state of (A) ubiquitin and (B) the 20+ charge state of myoglobin.
Figure 2
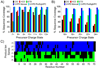
Sequence coverage produced by CID, HCD, ETD and UVPD as a function of precursor ion charge state for (A) ubiquitin and (B) myoglobin. (C) UVPD product ion array for the 10+ charge state of ubiquitin showing the distribution of cleavages throughout the protein. Green and blue areas indicate the observed cleavage for N-terminal and C-terminal product ions, respectively.
Figure 3
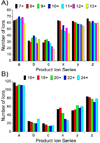
Number of each product ion type observed as function of precursor ion charge state for (A) ubiquitin and (B) myoglobin.
Figure 4
Crystal structure (PDB ID 3NTP) of Pin1 (top left) showing the locations of C57 and C113, which are observed in non-oxidized (C57, yellow) and oxidized (C113, red) forms, respectively. Mass spectrum of intact Pin1 (top right) showing doubly and triply oxidized species as the most abundant. Zoomed-in section of a 193 nm UVPD mass spectrum (bottom) of the 22+ charge state species, deconvolved to singly protonated species. Apostrophe (’) indicates the neutral loss of water.
Similar articles
- Thorough Performance Evaluation of 213 nm Ultraviolet Photodissociation for Top-down Proteomics.
Fornelli L, Srzentić K, Toby TK, Doubleday PF, Huguet R, Mullen C, Melani RD, Dos Santos Seckler H, DeHart CJ, Weisbrod CR, Durbin KR, Greer JB, Early BP, Fellers RT, Zabrouskov V, Thomas PM, Compton PD, Kelleher NL. Fornelli L, et al. Mol Cell Proteomics. 2020 Feb;19(2):405-420. doi: 10.1074/mcp.TIR119.001638. Epub 2019 Dec 30. Mol Cell Proteomics. 2020. PMID: 31888965 Free PMC article. - High-Throughput Analysis of Intact Human Proteins Using UVPD and HCD on an Orbitrap Mass Spectrometer.
Cleland TP, DeHart CJ, Fellers RT, VanNispen AJ, Greer JB, LeDuc RD, Parker WR, Thomas PM, Kelleher NL, Brodbelt JS. Cleland TP, et al. J Proteome Res. 2017 May 5;16(5):2072-2079. doi: 10.1021/acs.jproteome.7b00043. Epub 2017 Apr 19. J Proteome Res. 2017. PMID: 28412815 Free PMC article. - High-throughput database search and large-scale negative polarity liquid chromatography-tandem mass spectrometry with ultraviolet photodissociation for complex proteomic samples.
Madsen JA, Xu H, Robinson MR, Horton AP, Shaw JB, Giles DK, Kaoud TS, Dalby KN, Trent MS, Brodbelt JS. Madsen JA, et al. Mol Cell Proteomics. 2013 Sep;12(9):2604-14. doi: 10.1074/mcp.O113.028258. Epub 2013 May 21. Mol Cell Proteomics. 2013. PMID: 23695934 Free PMC article. - Ultraviolet Photodissociation Mass Spectrometry for Analysis of Biological Molecules.
Brodbelt JS, Morrison LJ, Santos I. Brodbelt JS, et al. Chem Rev. 2020 Apr 8;120(7):3328-3380. doi: 10.1021/acs.chemrev.9b00440. Epub 2019 Dec 18. Chem Rev. 2020. PMID: 31851501 Free PMC article. Review. - Pin1 flips Alzheimer's switch.
Etzkorn FA. Etzkorn FA. ACS Chem Biol. 2006 May 23;1(4):214-6. doi: 10.1021/cb600171g. ACS Chem Biol. 2006. PMID: 17163675 Review.
Cited by
- Comparison of Ultraviolet Photodissociation and Collision Induced Dissociation of Adrenocorticotropic Hormone Peptides.
Robotham SA, Brodbelt JS. Robotham SA, et al. J Am Soc Mass Spectrom. 2015 Sep;26(9):1570-9. doi: 10.1007/s13361-015-1186-y. Epub 2015 Jun 30. J Am Soc Mass Spectrom. 2015. PMID: 26122515 - Large-scale Top-down Proteomics Using Capillary Zone Electrophoresis Tandem Mass Spectrometry.
McCool EN, Lubeckyj R, Shen X, Kou Q, Liu X, Sun L. McCool EN, et al. J Vis Exp. 2018 Oct 24;(140):58644. doi: 10.3791/58644. J Vis Exp. 2018. PMID: 30417888 Free PMC article. - Proteoforms expand the world of microproteins and short open reading frame-encoded peptides.
Cassidy L, Kaulich PT, Tholey A. Cassidy L, et al. iScience. 2023 Jan 27;26(2):106069. doi: 10.1016/j.isci.2023.106069. eCollection 2023 Feb 17. iScience. 2023. PMID: 36818287 Free PMC article. Review. - Characterization of the T4 gp32-ssDNA complex by native, cross-linking, and ultraviolet photodissociation mass spectrometry.
Blevins MS, Walker JN, Schaub JM, Finkelstein IJ, Brodbelt JS. Blevins MS, et al. Chem Sci. 2021 Sep 23;12(41):13764-13776. doi: 10.1039/d1sc02861h. eCollection 2021 Oct 27. Chem Sci. 2021. PMID: 34760161 Free PMC article. - Trapped Ion Mobility Spectrometry, Ultraviolet Photodissociation, and Time-of-Flight Mass Spectrometry for Gas-Phase Peptide Isobars/Isomers/Conformers Discrimination.
Miller SA, Jeanne Dit Fouque K, Ridgeway ME, Park MA, Fernandez-Lima F. Miller SA, et al. J Am Soc Mass Spectrom. 2022 Jul 6;33(7):1267-1275. doi: 10.1021/jasms.2c00091. Epub 2022 Jun 5. J Am Soc Mass Spectrom. 2022. PMID: 35658468 Free PMC article.
References
- Duncan MW, Aebersold R, Caprioli RM. Nat Biotech. 2010;28:659–664. - PubMed
- Kelleher NL, Lin HY, Valaskovic GA, Aaserud DJ, Fridriksson EK, McLafferty FW. J. Am. Chem. Soc. 1999;121:806–812.
- McLafferty FW, Breuker K, Jin Mi, Han Xuemei, Infusini G, Jiang Honghai, Kong Xianglei, Begley TP. FEBS Journal. 2007;274:6256–6268. - PubMed
- Zhou H, Ning Z, E. Starr A, Abu-Farha M, Figeys D. Anal. Chem. 2011;84:720–734. - PubMed
- Little DP, Speir JP, Senko MW, O’Connor PB, McLafferty FW. Anal. Chem. 1994;66:2809–2815. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous