SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants - PubMed (original) (raw)
SigB is a dominant regulator of virulence in Staphylococcus aureus small-colony variants
Gabriel Mitchell et al. PLoS One. 2013.
Abstract
Staphylococcus aureus small-colony variants (SCVs) are persistent pathogenic bacteria characterized by slow growth and, for many of these strains, an increased ability to form biofilms and to persist within host cells. The virulence-associated gene expression profile of SCVs clearly differs from that of prototypical strains and is often influenced by SigB rather than by the agr system. One objective of this work was to confirm the role of SigB in the control of the expression of virulence factors involved in biofilm formation and intracellular persistence of SCVs. This study shows that extracellular proteins are involved in the formation of biofilm by three SCV strains, which, additionally, have a low biofilm-dispersing activity. It was determined that SigB activity modulates biofilm formation by strain SCV CF07-S and is dominant over that of the agr system without being solely responsible for the repression of proteolytic activity. On the other hand, the expression of fnbA and the control of nuclease activity contributed to the SigB-dependent formation of biofilm of this SCV strain. SigB was also required for the replication of CF07-S within epithelial cells and may be involved in the colonization of lungs by SCVs in a mouse infection model. This study methodically investigated SigB activity and associated mechanisms in the various aspects of SCV pathogenesis. Results confirm that SigB activity importantly influences the production of virulence factors, biofilm formation and intracellular persistence for some clinical SCV strains.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
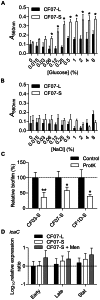
Figure 1. Formation of biofilms by SCVs may involve extracellular proteins.
The extent of biofilm production was measured by crystal violet staining (A560 nm) of normal CF07-L and SCV CF07-S strains as a function of glucose (A) and NaCl (B) concentrations following a 48-h incubation period. Statistically significant differences between strains are indicated for each concentration of glucose and NaCl (P<0.05; two-way ANOVA with Bonferonni's posttest, n = 3). (C) Susceptibility of SCVs' biofilms to treatment with proteinase K. Statistically significant differences between control and treated conditions are indicated (*, P<0.05; **, P<0.01; unpaired t test, n = 3). Results are normalized according to the control condition for each strain. (D) Expression ratio of the icaC gene as a function of growth for strains CF07-L, CF07-S and CF07-S in the presence of menadione, which restores normal growth. Results are expressed according to CF07-L in the early exponential phase of growth. No statistically significant difference was revealed between conditions for each growth phase (ANOVA with Dunnett's posttest, n = 3–4). Results are expressed as means with standard deviations.
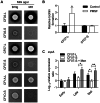
Figure 2. SCVs may have low biofilm-dispersing proteolytic activity.
(A) The proteolytic activities of normal (CF03-L, CF07-L and CF1A-L) and SCV (CF03-S, CF07-S and CF1D-S) strains were evaluated on BHI supplemented with glucose (BHIg) or MH milk-agar after 24 and 48 h, respectively. (B) Relative biofilm formation of CF07-L and CF07-S in the presence of the serine-protease inhibitor PMSF following 48 h of incubation. Results are normalized according to the control condition for each strain. A statistically significant difference is indicated between control and treated CF07-L (*, P<0.05; unpaired t test, n = 3). (C) Expression ratio of the sspA gene as a function of growth for strains CF07-L, CF07-S and CF07-S in the presence of menadione. Results are expressed according to CF07-L in the early exponential phase of growth. Statistically significant differences to CF07-S are indicated for each growth phase (*, P<0.05; **, P<0.01; ANOVA with Dunnett's posttest, n = 3–4). Results are expressed as means with standard deviations.
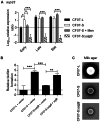
Figure 3. SigB modulates biofilm formation in SCV CF07-S but is not solely responsible for the repression of proteolysis.
(A) Expression ratio of the asp23 gene as a function of growth for strains CF07-L, CF07-S, CF07-S in the presence of menadione and CF07-SΔ_sigB_. Results are expressed according to CF07-L in the early exponential phase of growth. Statistically significant differences relative to CF07-S are indicated for each growth phase (*, P<0.05; ***, P<0.001; ANOVA with Dunnett's posttest, n = 3–4). (B) Relative biofilm formation of CF07-L, CF07-S and CF07-SΔ_sigB_ carrying the empty vector (pFM1) or the sigB expression vector (pFM2) following 48 h of incubation in the presence of 0.25 µM CdCl2. Statistically significant differences are indicated (**, P<0.01; ***, P<0.001; ANOVA with Tuckey's posttest, n = 3). Results are expressed as means with standard deviations. (C) Proteolytic activity of CF07-L, CF07-S and CF07-SΔ_sigB_ on BHIg milk-agar following 48 h of incubation.
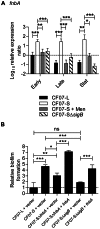
Figure 4. The agr system is influenced by SigB and modulates hemolysis and biofilm formation in SCV CF07-S.
Expression ratio of RNAIII (A) and the hla gene (B) as a function of growth for strains CF07-L, CF07-S, CF07-S in the presence of menadione and CF07-SΔ_sigB_. Results are expressed according to CF07-L in the early exponential phase of growth. Statistically significant differences to CF07-S are indicated for each growth phase (*, P<0.05; **, P<0.01; ***, P<0.001; ANOVA with Dunnett's posttest, n = 3–6). (C) Hemolytic activity of CF07-L, CF07-S, CF07-SΔ_sigB_ and CF07-SΔ_sigB_ carrying the empty vector (pFM1) or the sigB expression vector (pFM2) following 48 h of incubation on blood-agar plates supplemented with 0.25 µM CdCl2. (D) Relative biofilm formation of CF07-L, CF07-S and CF07-SΔ_sigB_ carrying the empty vector (pFM1) or the RNAIII expression vector (pFM4) following 48 h of incubation in the presence of 0.12 µM CdCl2. Statistically significant differences are indicated (ns, non statistically significant; *, P<0.05; **, P<0.01; ***, P<0.001; ANOVA with Tuckey's posttest, n = 3). (E) Expression ratio of the asp23 gene for CF07-S carrying the empty vector (pFM1) or the RNAIII expression vector (pFM4) grown to mid-exponential phase in the presence of 0.12 µM CdCl2. No statistically significant difference was revealed by an unpaired t test (n = 3). Results are expressed as means with standard deviations.
Figure 5. The SigB-dependent expression of FnBPA contributes to biofilm formation in SCV CF07-S.
(A) Expression ratio of the fnbA gene as a function of growth for strains CF07-L, CF07-S, CF07-S in the presence of menadione and CF07-SΔ_sigB_. Results are expressed according to CF07-L in the early exponential phase of growth. Statistically significant differences to CF07-S are indicated for each growth phase (*, P<0.05; **, P<0.01; ***, P<0.001; ANOVA with Dunnett's posttest, n = 4–9). (B) Relative biofilm formation of CF07-L, CF07-S, CF07-SΔ_fnbA_ and CF07-SΔ_sigB_ carrying the empty vector (pFM1) or the fnbA expression vector (pFM3) following 48 h of incubation in the presence of 0.25 µM CdCl2. Relevant statistically significant differences are indicated (ns, non statistically significant; *, P<0.05; **, P<0.01; ***, P<0.001; ANOVA with Tuckey's posttest, n = 3). Results are expressed as means with standard deviations.
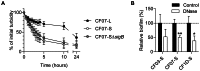
Figure 6. Extracellular DNA may be a component of the biofilm formed by SCVs.
(A) Autolysis of the CF07-L, CF07-S and CF07-SΔ_sigB_ strains as a function of time. Results are expressed as percentages of the initial turbidity for each condition. (B) Susceptibility of SCVs' biofilms to treatment with DNase I. Statistically significant differences between control and treated conditions are indicated (*, P<0.05; **, P<0.01; unpaired t test, n = 3–4). Results are normalized according to control condition for each strain. Results are expressed as means with standard deviations.
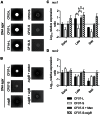
Figure 7. The nuclease activity of SCV CF07-S is repressed through a SigB-dependent mechanism.
(A) The nuclease activity of normal (CF03-L, CF07-L and CF1A-L) and SCV (CF03-S, CF07-S and CF1D-S) strains was evaluated on DNA agar following 24 h of incubation. (B) The nuclease activity of CF07-L, CF07-S, CF07-SΔ_sigB_ and CF07-SΔ_sigB_ carrying the empty vector (pFM1) or the sigB expression vector (pFM2) was evaluated on DNA agar following 48 h of incubation in the presence of 0.25 µM CdCl2. Expression ratios of nuc1 (A) and nuc2 (B) genes as a function of growth for strains CF07-L, CF07-S, CF07-S in the presence of menadione and CF07-SΔ_sigB_. Results are expressed according to CF07-L in the early exponential phase of growth. Statistically significant differences to CF07-S are indicated for each growth phase (*, P<0.05; ANOVA with Dunnett's posttest, n = 3–4). Results are expressed as means with standard deviations.
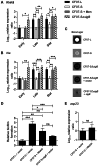
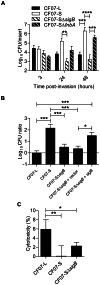
Figure 8. SigB is required for the replication of SCV CF07-S within CF cells.
(A) Infection kinetics of shCFTR Calu-3 cells (i.e., CF-like cells) by strains CF07-L, CF07-S, CF07-SΔ_sigB_ and CF07-SΔ_fnbA_. Statistically significant differences in CFU/insert recovered from infected cells are indicated (**, P<0.01; ***, P<0.001 ; ****, P<0.0001 ; two-way ANOVA with Bonferonni's posttest, n = 3–7). (B) CFU ratios recovered from cells infected with CF07-L, CF07-S, CF07-SΔ_sigB_ and CF07-SΔ_sigB_ carrying the empty vector (pFM1) or the sigB expression vector (pFM2) 48 h post-invasion. For cells infected with strains carrying a vector, the post-invasion media was supplemented with 0.25 µM CdCl2. Statistically significant differences between the CFU ratios recovered from cells infected with the different strains are indicated (*, P<0.05; ***, P<0.001; ANOVA with Tuckey's posttest, n = 3–7). (C) Host cell lysis was evaluated 48 h following invasion with CF07-L, CF07-S and CF07-SΔ_sigB_ by performing LDH cytotoxicity assays. Statistically significant differences are indicated (*, P<0.05; **, P<0.01; ANOVA with Tuckey's posttest, n = 4). Results are expressed as means with standard deviations.
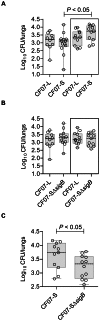
Figure 9. SigB provides better fitness to SCV CF07-S in a mouse pulmonary model of infection.
(A) CFU recovered from mouse lungs infected with CF07-L, CF07-S or a combination of both strains (boxed results) 48 h post-inoculation. A statistically significant difference is indicated (*, P<0.05; Kruskal-Wallis with a Dunn's posttest, n = 10–13). (B) CFU recovered from mouse lungs infected with CF07-L, CF07-SΔ_sigB_ or a combination of both strains (boxed results) 48 h post-inoculation. No statistically significant difference was revealed by a Kruskal-Wallis test followed by a Dunn's posttest (n = 10–11). (C) CFU recovered from mouse lungs infected with either CF07-S or CF07-SΔ_sigB_ during a co-infection with the normal strain CF07-L 48 h post-inoculation. A statistically significant difference is indicated (*, P<0.05; Mann-Whitney test, n = 11). For a given experiment, the quantity of bacteria in the mixed inoculum was equivalent to the sum of each inoculums prepared for individual strains. The CFU content was evaluated by plating logarithmic dilutions of homogenates on TSA and enumerating normal and SCV colonies.
Similar articles
- Transcription of virulence factors in Staphylococcus aureus small-colony variants isolated from cystic fibrosis patients is influenced by SigB.
Moisan H, Brouillette E, Jacob CL, Langlois-Bégin P, Michaud S, Malouin F. Moisan H, et al. J Bacteriol. 2006 Jan;188(1):64-76. doi: 10.1128/JB.188.1.64-76.2006. J Bacteriol. 2006. PMID: 16352822 Free PMC article. - A role for sigma factor B in the emergence of Staphylococcus aureus small-colony variants and elevated biofilm production resulting from an exposure to aminoglycosides.
Mitchell G, Brouillette E, Séguin DL, Asselin AE, Jacob CL, Malouin F. Mitchell G, et al. Microb Pathog. 2010 Jan;48(1):18-27. doi: 10.1016/j.micpath.2009.10.003. Epub 2009 Oct 13. Microb Pathog. 2010. PMID: 19825410 - Sigma Factor SigB Is Crucial to Mediate Staphylococcus aureus Adaptation during Chronic Infections.
Tuchscherr L, Bischoff M, Lattar SM, Noto Llana M, Pförtner H, Niemann S, Geraci J, Van de Vyver H, Fraunholz MJ, Cheung AL, Herrmann M, Völker U, Sordelli DO, Peters G, Löffler B. Tuchscherr L, et al. PLoS Pathog. 2015 Apr 29;11(4):e1004870. doi: 10.1371/journal.ppat.1004870. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25923704 Free PMC article. - Staphylococcus aureus dynamically adapts global regulators and virulence factor expression in the course from acute to chronic infection.
Tuchscherr L, Löffler B. Tuchscherr L, et al. Curr Genet. 2016 Feb;62(1):15-7. doi: 10.1007/s00294-015-0503-0. Epub 2015 Jun 30. Curr Genet. 2016. PMID: 26123224 Review. - Small colony variants (SCVs) of Staphylococcus aureus--a bacterial survival strategy.
Kahl BC. Kahl BC. Infect Genet Evol. 2014 Jan;21:515-22. doi: 10.1016/j.meegid.2013.05.016. Epub 2013 May 27. Infect Genet Evol. 2014. PMID: 23722021 Review.
Cited by
- Subpopulations in Strains of Staphylococcus aureus Provide Antibiotic Tolerance.
Mashayamombe M, Carda-Diéguez M, Mira A, Fitridge R, Zilm PS, Kidd SP. Mashayamombe M, et al. Antibiotics (Basel). 2023 Feb 17;12(2):406. doi: 10.3390/antibiotics12020406. Antibiotics (Basel). 2023. PMID: 36830316 Free PMC article. Review. - Prediction and validation of novel SigB regulon members in Bacillus subtilis and regulon structure comparison to Bacillales members.
Yeak KYC, Boekhorst J, Wels M, Abee T, Wells-Bennik MHJ. Yeak KYC, et al. BMC Microbiol. 2023 Jan 18;23(1):17. doi: 10.1186/s12866-022-02700-0. BMC Microbiol. 2023. PMID: 36653740 Free PMC article. - The Agr quorum-sensing system regulates fibronectin binding but not hemolysis in the absence of a functional electron transport chain.
Pader V, James EH, Painter KL, Wigneshweraraj S, Edwards AM. Pader V, et al. Infect Immun. 2014 Oct;82(10):4337-47. doi: 10.1128/IAI.02254-14. Epub 2014 Aug 4. Infect Immun. 2014. PMID: 25092909 Free PMC article. - Staphylococcus aureus Small Colony Variants (SCVs): a road map for the metabolic pathways involved in persistent infections.
Proctor RA, Kriegeskorte A, Kahl BC, Becker K, Löffler B, Peters G. Proctor RA, et al. Front Cell Infect Microbiol. 2014 Jul 28;4:99. doi: 10.3389/fcimb.2014.00099. eCollection 2014. Front Cell Infect Microbiol. 2014. PMID: 25120957 Free PMC article. Review. - Clinical Significance and Pathogenesis of Staphylococcal Small Colony Variants in Persistent Infections.
Kahl BC, Becker K, Löffler B. Kahl BC, et al. Clin Microbiol Rev. 2016 Apr;29(2):401-27. doi: 10.1128/CMR.00069-15. Clin Microbiol Rev. 2016. PMID: 26960941 Free PMC article. Review.
References
- Talbot GH, Bradley J, Edwards JE, Gilbert D, Scheld M, et al. (2006) Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis 42: 657–668. - PubMed
- Novick RP (2003) Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48: 1429–1449. - PubMed
- Novick RP, Geisinger E (2008) Quorum sensing in staphylococci. Annu Rev Genet 42: 541–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study was supported by a grant from Cystic Fibrosis Canada (http://www.cysticfibrosis.ca/en/index.php).GM was the recipient of an Alexander-Graham-Bell Graduate Scholarship from the Natural Science and Engineering Research Council of Canada (http://www.nserc-crsng.gc.ca) and received a doctoral research scholarship from the Fonds québécois de la recherche sur la nature et les technologies (http://www.fqrnt.gouv.qc.ca) during the course of this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources