Amphiregulin mediates progesterone-induced mammary ductal development during puberty - PubMed (original) (raw)
- PMID: 23705924
- PMCID: PMC3738150
- DOI: 10.1186/bcr3431
Amphiregulin mediates progesterone-induced mammary ductal development during puberty
Mark D Aupperlee et al. Breast Cancer Res. 2013.
Abstract
Introduction: Puberty is a period of increased susceptibility to factors that cause increased breast cancer risk in adulthood. Mammary end buds (EBs) that develop during puberty are believed to be the targets of breast cancer initiation. Whereas the role of estrogen (E) has been extensively studied in pubertal mammary gland development, the role of progesterone (P) during puberty is less defined.
Methods: Pubertal and prepubertal ovariectomized mice were treated with vehicle control (C), E, P, or E+P. Mammary glands from these mice were analyzed for changes in morphology, proliferation, and expression of the downstream targets amphiregulin (AREG) and receptor activator of NF-κB ligand (RANKL).
Results: P, acting specifically through the progesterone receptor, induced increases in mammary gland proliferation and EB formation that were associated with increased AREG expression in ducts and EBs. E, acting specifically through the estrogen receptor, produced similar responses also mediated by AREG. Blocking AREG action by treatment with an EGFR inhibitor completely abrogated the effect of P on EB formation and proliferation and significantly reduced proliferation within ducts. P also increased expression of RANKL, primarily in ducts. Treatment with RANK-Fc, an inhibitor of RANKL, reduced P-dependent proliferation in ducts and to a lesser extent in EB, but did not cause EB regression.
Conclusions: These results demonstrate a novel P-specific effect through AREG to cause EB formation and proliferation in the developing mammary gland both before and during puberty. Thus, hormones and/or factors in addition to E that upregulate AREG can promote mammary gland development and have the potential to affect breast cancer risk associated with pubertal mammary gland development.
Figures
Figure 1
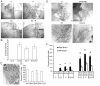
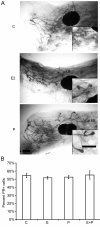
Both 17-β-estradiol and progesterone induce similar morphologic and proliferative responses in the pubertal mammary gland. Pubertal 4-week-old BALB/c mice were OVX, allowed to recover for 3 weeks, and then treated for 5 days with vehicle control (C), E2, P, or E2+P, as described in the Materials and Methods section. (A) Morphologic response to 5d C, E2, P, or E2+P. Note lack of EBs in controls and similar presence of EBs in E2-, P-, and E2+P-treated glands. Scale bar, 1 mm. (B) End bud (EB) quantitation. Values represent the mean ± SEM number of EBs in a number 4 mammary gland (n = 3 mice). (C) Morphologic response to 5d P in the adult 17-week-old BALB/c OVX mammary gland; note presence of extensive side branching. Scale bar, 1 mm. (D) Immunofluorescent detection of PR expression in luminal cells. The values represent the mean ± SEM percentage PR-positive cells (n = 3 animals per treatment). The percentage PR-positive cells in 5-day P-treated BALB/c mice was less than control (*P < 0.05). (E) Inhibition of EB formation by E2+ ICI 182,780 and P+RU486. Scale bar, 1 mm. (F) Proliferation analysis by dual immunofluorescent detection of BrdU and PR. The total percentages BrdU-positive cells and BrdU-positive cells co-expressing PR in ducts and EBs are presented. The values represent the mean ± SEM (n = 3 animals per treatment). The percentage of BrdU-positive luminal epithelial cells in ducts after E2, P, or E2+P treatment was greater than control (*P < 0.05). The percentage of BrdU-positive cells was greater in EBs than in ducts after E2, P, or E2+P treatment (#P < 0.05).
Figure 2
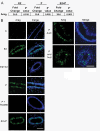
Both 17-β-estradiol and progesterone regulate amphiregulin through their cognate receptors in the pubertal mammary gland. Pubertal 4-week-old BALB/c mice were OVX, allowed to recover for 3 weeks, and then treated for 5 days with vehicle control (C), E2, P, or E2+P, as described in the Materials and Methods section. (A) RT-PCR analysis of amphiregulin (AREG) in response to hormone treatments. Fold change is relative to control treatment. (B) Immunofluorescent detection of AREG (green) in ducts after E2, E2+ICI, P, P+RU486, and after P treatment in both ducts and end buds. Nuclei were counterstained with DAPI (blue). Scale bar, 25 μm.
Figure 3
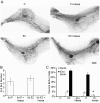
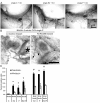
Iressa inhibits both 17-β-estradiol and progesterone-induced proliferation and EB formation. Pubertal 4-week-old BALB/c mice were OVX, allowed to recover for 3 weeks, and then treated for 5 days with vehicle control (C), E2 +/- Iressa (300 mg/kg), or P +/- Iressa, as described in the Materials and Methods section. (A) EB formation after 5 days E2, E2+Iressa, P, or P+Iressa. Note the absence of EBs in Iressa-treated mammary glands. Scale bar, 2 mm. (B) End bud quantitation. Values represent the mean ± SEM number of end buds in a number 4 mammary gland (n = 5 mice). (C) The percentage of proliferating cells in ducts and EBs was determined by immunofluorescence detection of BrdU incorporation. P+Iressa treatment reduced proliferation in ducts relative to P treatment (*P< 0.05).
Figure 4
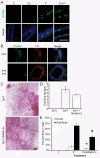
Progesterone-induced RANKL expression contributes to the proliferative response in the pubertal mammary gland. Pubertal 4-week-old BALB/c mice were OVX, allowed to recover for 3 weeks, and then treated for 5 days with vehicle control (C), E2, P, or E2+P, as described in the Materials and Methods section. (A) Immunofluorescent detection of RANKL (green) in P and E2+P-treated mammary glands. (B) Dual immunofluorescent detection of RANKL (green) and PR (red) expression in representative images of an E2+P-treated duct and end bud. RANKL was most strongly expressed in ducts. Nuclei (A, B) were counterstained with DAPI (blue). Scale bars, 25 μm. (C) Effect of 5d P versus 5-day P+RANK-Fc on EB formation; note lack of significant EB regression with RANK-Fc treatment. Scale bar, 1 mm.(D) End-bud quantitation. Values represent the mean ± SEM number of end buds in the thoracic mammary gland (n = 3 mice). (E) The percentage of proliferating cells in ducts and EBs was determined by immunofluorescent detection of BrdU incorporation after C, P, and P+RANK-Fc treatment. P+RANK-Fc treatment had fewer proliferating cells in ducts than in P treatment (*P < 0.05). P+RANK-Fc treatment had fewer proliferating cells in EB than in P treatment (#P < 0.05).
Figure 5
Both 17-β-estradiol and progesterone induce morphologic responses in the prepubertal mammary gland. Prepubertal 3-week-old BALB/c mice were OVX, allowed to recover for 2 weeks, and then treated once with vehicle control (C), E2, P, or E2+P, as described in the Materials and Methods section. **(A)**End bud (EB) formation in response to C, E2, and P. Inset shows higher magnification of EB. Note similar EB formation after E2 or P treatments. Scale bar, 1 mm. (B) PR expression. Immunofluorescent detection of PR-positive luminal cells. The values represent the mean ± SEM percentage of PR-positive cells (n = 3 animals per treatment).
Figure 6
Both 17-β-estradiol and progesterone-induced responses in the prepubertal mammary gland occur through their cognate receptors. **(A)**Representative whole mounts from prepubertal 3-week-old BALB/c mice that were OVX, allowed to recover for 2 weeks, and then treated once with vehicle control (C) + ICI 182,780 (ICI), E2 + ICI, or P + ICI, as described in the Materials and Methods section. Scale bar, 1 mm. Inset shows higher magnification of end buds (EBs), which were present in only the P + ICI treatment group. Scale bar, 1 mm. (B) Effect of RU486 implant on P-induced EB formation. RU486 pellet implanted in the right mammary gland number 4, and control pellet implanted in the contralateral number 4 mammary gland. Implants are outlined with dashed lines and noted with an asterisk. Note stimulated EB in control L4 gland and nonstimulated duct ends (arrows). Scale bar, 2 mm. (C) Proliferation analysis by dual immunofluorescence detection of BrdU and PR. Total percentage BrdU-positive cells and percentage BrdU-positive cells coexpressing PR in ducts and end buds after C, E2, P, or E2+P treatment are presented. The percentage of BrdU-positive cells in ducts increased in response to E2, P, or E2+P treatment compared with control (*P< 0.05). The percentage of BrdU-positive cells increased in end buds compared with ducts in E2, P, or E2+P (#P < 0.05).
Figure 7
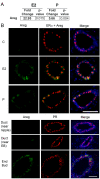
17-β-Estradiol regulates amphiregulin, and progesterone regulates both amphiregulin and RANKL in the prepubertal mammary gland. Prepubertal 3-week-old BALB/c mice were OVX, allowed to recover for 2 weeks, and treated once with vehicle control (C), E2, or P, as described in the Materials and Methods section. (A) Expression analysis of amphiregulin (AREG) by RT-PCR. Fold change is relative to control treatment. (B) Dual immunofluorescence detection of AREG (green) and either ERα (red, on left) or PR (red, on right). AREG expression increased in response to E2 or P in ERα-expressing cells and was most strongly expressed in end buds in response to P treatment. Nuclei were counterstained with DAPI (blue). Scale bar, 25 μm.
Similar articles
- Amphiregulin mediates estrogen, progesterone, and EGFR signaling in the normal rat mammary gland and in hormone-dependent rat mammary cancers.
Kariagina A, Xie J, Leipprandt JR, Haslam SZ. Kariagina A, et al. Horm Cancer. 2010 Oct;1(5):229-44. doi: 10.1007/s12672-010-0048-0. Epub 2010 Nov 23. Horm Cancer. 2010. PMID: 21258428 Free PMC article. - Epidermal growth factor receptor (EGFR) signaling is a key mediator of hormone-induced leukocyte infiltration in the pubertal female mammary gland.
Aupperlee MD, Zhao Y, Tan YS, Leipprandt JR, Bennett J, Haslam SZ, Schwartz RC. Aupperlee MD, et al. Endocrinology. 2014 Jun;155(6):2301-13. doi: 10.1210/en.2013-1933. Epub 2014 Apr 2. Endocrinology. 2014. PMID: 24693965 Free PMC article. - Loss of amphiregulin reduces myoepithelial cell coverage of mammary ducts and alters breast tumor growth.
Mao SPH, Park M, Cabrera RM, Christin JR, Karagiannis GS, Oktay MH, Zaiss DMW, Abrams SI, Guo W, Condeelis JS, Kenny PA, Segall JE. Mao SPH, et al. Breast Cancer Res. 2018 Oct 26;20(1):131. doi: 10.1186/s13058-018-1057-0. Breast Cancer Res. 2018. PMID: 30367629 Free PMC article. - Amphiregulin: role in mammary gland development and breast cancer.
McBryan J, Howlin J, Napoletano S, Martin F. McBryan J, et al. J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):159-69. doi: 10.1007/s10911-008-9075-7. Epub 2008 Apr 9. J Mammary Gland Biol Neoplasia. 2008. PMID: 18398673 Review. - The ADAM17-amphiregulin-EGFR axis in mammary development and cancer.
Sternlicht MD, Sunnarborg SW. Sternlicht MD, et al. J Mammary Gland Biol Neoplasia. 2008 Jun;13(2):181-94. doi: 10.1007/s10911-008-9084-6. Epub 2008 May 10. J Mammary Gland Biol Neoplasia. 2008. PMID: 18470483 Free PMC article. Review.
Cited by
- Single-cell and spatial analyses reveal a tradeoff between murine mammary proliferation and lineage programs associated with endocrine cues.
Gray GK, Girnius N, Kuiken HJ, Henstridge AZ, Brugge JS. Gray GK, et al. Cell Rep. 2023 Oct 31;42(10):113293. doi: 10.1016/j.celrep.2023.113293. Epub 2023 Oct 17. Cell Rep. 2023. PMID: 37858468 Free PMC article. - ERBB Receptors and Their Ligands in the Developing Mammary Glands of Different Species: Fifteen Characters in Search of an Author.
Morato A, Accornero P, Hovey RC. Morato A, et al. J Mammary Gland Biol Neoplasia. 2023 May 23;28(1):10. doi: 10.1007/s10911-023-09538-w. J Mammary Gland Biol Neoplasia. 2023. PMID: 37219601 Free PMC article. Review. - The molecular consequences of androgen activity in the human breast.
Raths F, Karimzadeh M, Ing N, Martinez A, Yang Y, Qu Y, Lee TY, Mulligan B, Devkota S, Tilley WT, Hickey TE, Wang B, Giuliano AE, Bose S, Goodarzi H, Ray EC, Cui X, Knott SRV. Raths F, et al. Cell Genom. 2023 Mar 8;3(3):100272. doi: 10.1016/j.xgen.2023.100272. eCollection 2023 Mar 8. Cell Genom. 2023. PMID: 36950379 Free PMC article. - Adolescent- and adult-initiated alcohol exposure in mice differentially promotes tumorigenesis and metastasis of breast cancer.
Xu M, Li H, Chen D, Wu H, Wen W, Xu H, Frank J, Chen G, Luo J. Xu M, et al. Alcohol Clin Exp Res (Hoboken). 2023 Feb;47(2):251-262. doi: 10.1111/acer.14986. Epub 2022 Dec 10. Alcohol Clin Exp Res (Hoboken). 2023. PMID: 36462938 Free PMC article. - High Levels of Progesterone Receptor B in MCF-7 Cells Enable Radical Anti-Tumoral and Anti-Estrogenic Effect of Progestin.
Bajalovic N, Or YZ, Woo ARE, Lee SH, Lin VCL. Bajalovic N, et al. Biomedicines. 2022 Aug 2;10(8):1860. doi: 10.3390/biomedicines10081860. Biomedicines. 2022. PMID: 36009407 Free PMC article.
References
- Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(6 Suppl):S18–24. - PubMed
- Russo IH, Russo J. Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12-dimethylbenz[a]anthracene. J Natl Cancer Inst. 1978;61:1439–1449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous