Immune sensing of DNA - PubMed (original) (raw)
Review
Immune sensing of DNA
Søren R Paludan et al. Immunity. 2013.
Abstract
Although it has been appreciated for some years that cytosolic DNA is immune stimulatory, it is only in the past five years that the molecular basis of DNA sensing by the innate immune system has begun to be revealed. In particular it has been described how DNA induces type I interferon, central in antiviral responses and a mediator of autoimmunity. To date more than ten cytosolic receptors of DNA have been proposed, but STING is a key adaptor protein for most DNA-sensing pathways, and we are now beginning to understand the signaling mechanisms for STING. In this review we describe the recent progress in understanding signaling mechanisms activated by DNA and the relevance of DNA sensing to pathogen responses and autoimmunity. We highlight new insights gained into how and why the immune system responds to both pathogen and self DNA and define important questions that now need to be addressed in the field of innate immune activation by DNA.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
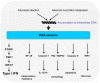
Figure 1. Cellular functions stimulated by DNA
Intracellular DNA is recognized by DNA sensors leading to activation of multiple pathways. The best characterized DNA-stimulated pathway is the one leading to activation of IRFs and induction of IFNs. Other well-characterized pathways activated by DNA recognition are the inflammatory NF-κB and inflammasome pathways, which stimulate expression of inflammatory genes and cleavage of pro-IL-1β and IL-18, respectively. Intracellular DNA also stimulates autophagy and different types of cell death.
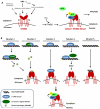
Figure 2. Immunostimulatory DNA and host sensors
DNA from microbes or the host has the potential to activate innate immune responses if delivered to the cytoplasm, and in some instances also the nucleus. DNA can end up in the cytoplasm through a variety of different pathways, and several different proteins have been proposed to function as PRR for DNA. The main DNA activated signaling pathways proceed through MyD88 for TLR9, DHX9 or DHX36, through ASC-caspase1 for AIM2, and via STING-TBK1-IRF3 for most other cytosolic IFN-inducing sensors.
Figure 3. Models for STING activation
(A) STING resides in the ER either as a monomer or more likely as a dimer (shown) in an autoinhibited state and is ‘activated’ by intracellular DNA, CDNs, and membrane perturbation. This leads to formation of an active STING dimer and mobilization to perinuclear vesicular structures where the C-terminal domain of STING serves as a platform for TBK1-mediated phosphorylation of IRF3. (B) Based on the current literature several models for STING ‘activation’ are possible. 1, DNA is sensed by a DNA sensor which initiates downstream signaling involving production of a second messenger (cGAMP) which binds to STING causing a conformational change essential for STING to recruit TBK1. 2, cGAS is the initial sensor of DNA and is activated by DNA binding to produce cGAMP, hence triggering STING activation as in (1). 3, DNA sensors directly interact with STING upon DNA binding and thereby stimulate the conformational change required for STING to recruit TBK1. 4, STING ‘activation’ involves other mechanisms such as redox-regulated covalent linkage of the monomers (Jin et al., 2011). 5, STING binds DNA directly and stimulates downstream signaling.
Similar articles
- Cytosolic DNA sensors regulating type I interferon induction.
Keating SE, Baran M, Bowie AG. Keating SE, et al. Trends Immunol. 2011 Dec;32(12):574-81. doi: 10.1016/j.it.2011.08.004. Epub 2011 Sep 21. Trends Immunol. 2011. PMID: 21940216 Review. - Regulation of RLR-mediated innate immune signaling--it is all about keeping the balance.
Eisenächer K, Krug A. Eisenächer K, et al. Eur J Cell Biol. 2012 Jan;91(1):36-47. doi: 10.1016/j.ejcb.2011.01.011. Epub 2011 Apr 9. Eur J Cell Biol. 2012. PMID: 21481967 Review. - A selective contribution of the RIG-I-like receptor pathway to type I interferon responses activated by cytosolic DNA.
Choi MK, Wang Z, Ban T, Yanai H, Lu Y, Koshiba R, Nakaima Y, Hangai S, Savitsky D, Nakasato M, Negishi H, Takeuchi O, Honda K, Akira S, Tamura T, Taniguchi T. Choi MK, et al. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17870-5. doi: 10.1073/pnas.0909545106. Epub 2009 Oct 1. Proc Natl Acad Sci U S A. 2009. PMID: 19805092 Free PMC article. - Innate immune recognition of DNA: A recent history.
Dempsey A, Bowie AG. Dempsey A, et al. Virology. 2015 May;479-480:146-52. doi: 10.1016/j.virol.2015.03.013. Epub 2015 Mar 26. Virology. 2015. PMID: 25816762 Free PMC article. Review. - Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing.
Chen Q, Sun L, Chen ZJ. Chen Q, et al. Nat Immunol. 2016 Sep 20;17(10):1142-9. doi: 10.1038/ni.3558. Nat Immunol. 2016. PMID: 27648547 Review.
Cited by
- Network representations of immune system complexity.
Subramanian N, Torabi-Parizi P, Gottschalk RA, Germain RN, Dutta B. Subramanian N, et al. Wiley Interdiscip Rev Syst Biol Med. 2015 Jan-Feb;7(1):13-38. doi: 10.1002/wsbm.1288. Epub 2015 Jan 27. Wiley Interdiscip Rev Syst Biol Med. 2015. PMID: 25625853 Free PMC article. Review. - HIV-1 Treated Patients with Undetectable Viral Loads have Lower Levels of Innate Immune Responses via Cytosolic DNA Sensing Systems Compared with Healthy Uninfected Controls.
Swaminathan S, Sui H, Adelsberger JW, Chen Q, Sneller M, Migueles SA, Kottilil S, Ober A, Jones S, Rehm CA, Lane HC, Imamichi T. Swaminathan S, et al. J AIDS Clin Res. 2014 Jun;5(6):315. doi: 10.4172/2155-6113.1000315. J AIDS Clin Res. 2014. PMID: 26023356 Free PMC article. - RKIP and TBK1 form a positive feedback loop to promote type I interferon production in innate immunity.
Gu M, Liu Z, Lai R, Liu S, Lin W, Ouyang C, Ye S, Huang H, Wang X. Gu M, et al. EMBO J. 2016 Dec 1;35(23):2553-2565. doi: 10.15252/embj.201694060. Epub 2016 Oct 17. EMBO J. 2016. PMID: 27753621 Free PMC article. - Type I interferon and lymphangiogenesis in the HSV-1 infected cornea - are they beneficial to the host?
Bryant-Hudson K, Conrady CD, Carr DJ. Bryant-Hudson K, et al. Prog Retin Eye Res. 2013 Sep;36:281-91. doi: 10.1016/j.preteyeres.2013.06.003. Epub 2013 Jul 19. Prog Retin Eye Res. 2013. PMID: 23876483 Free PMC article. Review. - The Effect of a TLR3 Agonist on Airway Allergic Inflammation and Viral Infection in Immunoproteasome-Deficient Mice.
Schaunaman N, Nichols T, Cervantes D, Hartsoe P, Ferrington DA, Chu HW. Schaunaman N, et al. Viruses. 2024 Aug 29;16(9):1384. doi: 10.3390/v16091384. Viruses. 2024. PMID: 39339860 Free PMC article.
References
- Aglipay JA, Lee SW, Okada S, Fujiuchi N, Ohtsuka T, Kwak JC, Wang Y, Johnstone RW, Deng C, Qin J, Ouchi T. A member of the Pyrin family, IFI16, is a novel BRCA1-associated protein involved in the p53-mediated apoptosis pathway. Oncogene. 2003;22:8931–8938. - PubMed
- Agrawal H, Jacob N, Carreras E, Bajana S, Putterman C, Turner S, Neas B, Mathian A, Koss MN, Stohl W, Kovats S, Jacob CO. Deficiency of Type I IFN Receptor in Lupus-Prone New Zealand Mixed 2328 Mice Decreases Dendritic Cell Numbers and Activation and Protects from Disease. Journal of Immunology. 2009;183:6021–6029. - PMC - PubMed
- Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H. Bacterial CpG-DNA and lipopolysaccharides activate Toll-like receptors at distinct cellular compartments. Eur. J. Immunol. 2002;32:1958–1968. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous