KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling - PubMed (original) (raw)
KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling
Abderrahmane Kaidi et al. Nature. 2013.
Retraction in
- Retraction Note: KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling.
Kaidi A, Jackson SP. Kaidi A, et al. Nature. 2019 Apr;568(7753):576. doi: 10.1038/s41586-019-1142-2. Nature. 2019. PMID: 30976101 Free PMC article.
Abstract
The detection of DNA lesions within chromatin represents a critical step in cellular responses to DNA damage. However, the regulatory mechanisms that couple chromatin sensing to DNA-damage signalling in mammalian cells are not well understood. Here we show that tyrosine phosphorylation of the protein acetyltransferase KAT5 (also known as TIP60) increases after DNA damage in a manner that promotes KAT5 binding to the histone mark H3K9me3. This triggers KAT5-mediated acetylation of the ATM kinase, promoting DNA-damage-checkpoint activation and cell survival. We also establish that chromatin alterations can themselves enhance KAT5 tyrosine phosphorylation and ATM-dependent signalling, and identify the proto-oncogene c-Abl as a mediator of this modification. These findings define KAT5 tyrosine phosphorylation as a key event in the sensing of genomic and chromatin perturbations, and highlight a key role for c-Abl in such processes.
Figures
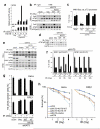
Figure 1. KAT5 tyrosine phosphorylation enhances its binding to H3K9me3
a, HeLa cells stably expressing Flag-KAT5 were exposed to IR then subjected to Flag immunoprecipitation and elution by Flag peptides. b, Binding of Flag-KAT5 to H3-derived peptide (residues 1-20) in which K9 is methylated (M) or unmethylated (U). Eluates from a were incubated with the M or U peptides immobilized on agarose beads; after extensive washing, samples were examined by SDS-PAGE and western blotting. Where indicated, KAT5 samples were treated with phosphatase before the binding assay. The input panel represents portions of KAT5 samples before the binding assays. c, Alignment of regions from KAT5 chromodomains of various species. d, Immunoprecipitations were performed with cell extracts from HeLa or RPE1 cells expressing Flag-KAT5 (wild type: WT; or Tyr-44 to Phe mutant: YF) then analyzed by western blotting with anti-phosphotyrosine antibody (pY). e, Time-course of Flag-KAT5 tyrosine phosphorylation after IR of HeLa or RPE1 cells followed by Flag- immunoprecipitation and western blotting. f, Time-course of endogenous KAT5 tyrosine phosphorylation after IR (arrow indicates the band corresponding to Tyr phosphorylated KAT5). g, Analysis of Flag-KAT (WT or YF) binding to H3-derived methylated peptide.
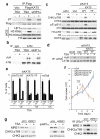
Figure 2. KAT5 Tyr phosphorylation promotes ATM activation, checkpoint signaling and cell survival after IR
a, Flag-KAT5 (WT or YF) was purified from HeLa cells before or after IR treatment. The effect of H3K9me3 peptide on Flag-KAT activity towards ATM or H4 was measured by in vitro acetylation followed by western blotting with anti-acetyl-lysine antibody and quantification. Data from three independent experiments are presented as mean ± SE. b, ATM acetylation was examined after immunoprecipitation followed by western blotting with anti-acetyl-lysine (AcK) antibody (IgG was used as negative control). Extracts were derived from HeLa or RPE1 cells that express siRNA resistant KAT5 (WT or YF) in which endogenous KAT5 was depleted by siRNA as indicated. c, Analysis of H4 acetylation on lysine 16 (H4K16ac) on the p73 promoter by chromatin immunoprecipitation (ChIP) in RPE1 cells that express siRNA resistant KAT5 (WT or YF) wherein endogenous KAT5 was siRNA depleted as indicated. Data are means from three independent experiments, each performed in duplicate, ± SD. d, Analysis of ATM auto-phosphorylation on Ser-1981 in the RPE1 cell complementation system. e, ATM-dependent signaling in HeLa and RPE1 cells expressing siRNA resistant KAT5 derivatives (endogenous KAT5 was siRNA depleted). Cells were examined 1 h after 3 Gy of IR. f, Cell cycle analyses of RPE1 cells expressing siRNA resistant KAT5 derivatives (endogenous KAT5 was siRNA depleted) at indicated times after 3 Gy IR. Results are means from three independent experiments ± SE. g, The G2/M DNA damage checkpoint was assessed in HeLa and RPE1 cells expressing siRNA resistant KAT5 derivatives (endogenous KAT5 was siRNA depleted), by measuring the percentage of cells positive for histone H3 phosphorylated at S10 (H3 pS10) by flow cytometry 2 h post IR (3 Gy). Results are means from three independent experiments ± SEM. h, Cell survival after IR was examined in HeLa and RPE1 cells expressing vector-only control or siRNA-resistant KAT5 constructs (endogenous KAT5 was siRNA depleted). Data represent averages from three independent experiments, each performed in duplicate ± SD.
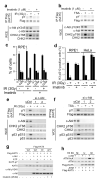
Figure 3. Chromatin alterations activate KAT5 phosphorylation and checkpoint signaling
a, Analysis of KAT5 tyrosine phosphorylation after chromatin alteration. RPE1 cells were treated with trichostatin A (TSA) for 5 h or siRNA depleted for heterochromatin protein 1α (HP1α), then analyzed for Flag-KAT tyrosine phosphorylation following immunoprecipitation and western blotting. The total cell extract (TCE) panel confirms efficacy of TSA treatment (increased H3 lysine 9 acetylation, H3K9ac) and HP1α depletion. b, RPE1 cells were TSA treated (5 h) or depleted for HP1α and analyzed for ATM Lys acetylation after immunoprecipitation and western blotting. c, RPE1 cells expressing siRNA-resistant KAT5 derivatives (endogenous KAT5 was siRNA depleted) were examined for ATM-mediated signaling after TSA treatment. d, RPE1 cells expressing siRNA-resistant KAT5 derivatives (endogenous KAT5 was siRNA depleted) were examined for ATM acetylation after TSA treatment (5 h) followed by ATM immunoprecipitation and western blotting with anti-AcK antibody. e, Flow cytometry analyses of RPE1 cells treated with TSA for 12 h. In the left and middle panels, cells were depleted for endogenous KAT5 and complemented with KAT5-WT or KAT5-YF; in the right panel, cells were pre-treated with ATMi (1 h) and left for an extra 12 h after TSA treatment. Results are means from three independent experiments ± SEM. f, Cell growth assay of RPE1 cells depleted for endogenous KAT5 and complemented with vector-only control, KAT5-WT or KAT5-YF. Twenty-four hours after siRNA transfection, 104 cells were seeded and allowed to recover for 24 h, after which cells were treated with TSA for 16 h. Cells were collected every 24 h and counted. Data from three independent experiments performed in duplicates are presented as mean ± SEM. g, MRE11 was depleted in RPE1 cells and checkpoint signaling was analyzed by western blotting after TSA treatment (left), HP1α depletion (middle), or IR exposure (right).
Figure 4. KAT5 chromatin binding promotes its phosphorylation
a, RPE1 cells were treated with okadaic acid (OA; 25 nM) for indicated times and Flag-KAT tyrosine phosphorylation was examined after immunoprecipitation and western blotting. b, KAT5 binding to H3K9me3 peptide protects it from dephosphorylation. Flag-KAT5 was incubated with H3-derived peptide either methylated (M) or unmethylated (U), after which samples were subjected to phosphatase treatment and analyzed by western blotting. c, KAT5 tyrosine phosphorylation was examined after chromatin fractionation of RPE1 cells expressing Flag-KAT5 and treated with IR (left panel) or TSA (right panel). S denotes soluble fractions after treatment with cytoskeleton (CSK) buffer, while C denotes chromatin fractions remaining after CSK treatment. d, Flag-based immunoprecipitations were performed on benzonase cell extracts prepared from HeLa cells expressing siRNA-resistant KAT5 (WT or YF) in which endogenous KAT5 was siRNA depleted before or after treating cells with IR. Elutes were analyzed by western blotting. e, Wild-type and chromodomain mutant Flag-KAT5 (WT and CD: Phe-43 to Ala and Tyr-47 to Ala substitutions) were analyzed for tyrosine phosphorylation after immunoprecipitation and western blotting of extracts derived from IR- (upper panel) or TSA- (lower panel) treated RPE1 cells. f, WT, CD and YF Flag-KAT5 were analyzed for tyrosine phosphorylation following immunoprecipitation and western blotting of extracts from mock- or OA-treated RPE1 cells.
Figure 5. c-Abl-dependent KAT5 tyrosine phosphorylation
a-b, RPE1 cells were pretreated with imatinib (1μM) for 3 h followed by exposure to IR or TSA; cell extracts were prepared 1 h after IR (a) or 5 h after TSA (b) treatments, and were analysed by immunoprecipitation and western blotting as indicated. c, Flow cytometry analyses of RPE1 cells exposed to IR in the presence or absence of imatinib. Cells were collected 8 h afterwards and analysed. Results are means from three independent experiments ± SE. d, Analysis of G2/M checkpoint in RPE1 and HeLa cells after exposure to IR in the presence or absence of imatinib. Cells were harvested 2 h after IR, stained with H3 pS10 antibody and analysed by flow cytometry. Data from three independent experiments are presented as mean ± SEM. e-f, RPE1 cells were transfected with either of two independent c-Abl targeting siRNAs and, 36 h later were exposed to IR (e) or TSA (f). Extracts were prepared after 1 h and were analysed by immunoprecipitation and western blotting. g, In vitro kinase assays were performed with recombinant c-Abl and purified Flag-KAT5 (WT or YF). Reactions were initiated by ATP addition, and samples were analyzed by western blotting to examine KAT5 tyrosine phosphorylation. h, KAT5 was purified from HeLa cells expressing Flag-KAT5. Purified KAT5 bound to beads was subjected to c-Abl-mediated phosphorylation. After washes, KAT5 was eluted and its activity towards ATM was assessed in the presence of K9 methylated (M) or unmethylated (U) H3 (1-20) peptide by using an AcK antibody.
Comment in
- DNA repair: A sensor for chromatin damage.
David R. David R. Nat Rev Mol Cell Biol. 2013 Jul;14(7):400-1. doi: 10.1038/nrm3603. Epub 2013 Jun 5. Nat Rev Mol Cell Biol. 2013. PMID: 23736680 No abstract available. - Kinases and chromatin structure: who regulates whom?
Miotto B. Miotto B. Epigenetics. 2013 Oct;8(10):1008-12. doi: 10.4161/epi.25909. Epub 2013 Aug 5. Epigenetics. 2013. PMID: 23917692 Free PMC article.
Similar articles
- Tip60: connecting chromatin to DNA damage signaling.
Sun Y, Jiang X, Price BD. Sun Y, et al. Cell Cycle. 2010 Mar 1;9(5):930-6. doi: 10.4161/cc.9.5.10931. Epub 2010 Mar 11. Cell Cycle. 2010. PMID: 20160506 Free PMC article. Review. - Regulation of TIP60 by ATF2 modulates ATM activation.
Bhoumik A, Singha N, O'Connell MJ, Ronai ZA. Bhoumik A, et al. J Biol Chem. 2008 Jun 20;283(25):17605-14. doi: 10.1074/jbc.M802030200. Epub 2008 Apr 8. J Biol Chem. 2008. PMID: 18397884 Free PMC article. - MYST family lysine acetyltransferase facilitates ataxia telangiectasia mutated (ATM) kinase-mediated DNA damage response in Toxoplasma gondii.
Vonlaufen N, Naguleswaran A, Coppens I, Sullivan WJ Jr. Vonlaufen N, et al. J Biol Chem. 2010 Apr 9;285(15):11154-61. doi: 10.1074/jbc.M109.066134. Epub 2010 Feb 16. J Biol Chem. 2010. PMID: 20159970 Free PMC article. - A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM.
Sun Y, Jiang X, Chen S, Fernandes N, Price BD. Sun Y, et al. Proc Natl Acad Sci U S A. 2005 Sep 13;102(37):13182-7. doi: 10.1073/pnas.0504211102. Epub 2005 Sep 2. Proc Natl Acad Sci U S A. 2005. PMID: 16141325 Free PMC article. - Mechanistic links between ATM and histone methylation codes during DNA repair.
Xu Y, Xu C, Price BD. Xu Y, et al. Prog Mol Biol Transl Sci. 2012;110:263-88. doi: 10.1016/B978-0-12-387665-2.00010-9. Prog Mol Biol Transl Sci. 2012. PMID: 22749149 Review.
Cited by
- The stress-responsive gene ATF3 regulates the histone acetyltransferase Tip60.
Cui H, Guo M, Xu D, Ding ZC, Zhou G, Ding HF, Zhang J, Tang Y, Yan C. Cui H, et al. Nat Commun. 2015 Apr 13;6:6752. doi: 10.1038/ncomms7752. Nat Commun. 2015. PMID: 25865756 Free PMC article. - Targeted Cancer Therapy Based on Acetylation and Deacetylation of Key Proteins Involved in Double-Strand Break Repair.
Wang X, Zhao J. Wang X, et al. Cancer Manag Res. 2022 Jan 22;14:259-271. doi: 10.2147/CMAR.S346052. eCollection 2022. Cancer Manag Res. 2022. PMID: 35115826 Free PMC article. Review. - CircRHOT1 restricts gastric cancer cell ferroptosis by epigenetically regulating GPX4.
Wang H, Breadner DA, Deng K, Niu J. Wang H, et al. J Gastrointest Oncol. 2023 Aug 31;14(4):1715-1725. doi: 10.21037/jgo-23-550. Epub 2023 Aug 30. J Gastrointest Oncol. 2023. PMID: 37720433 Free PMC article. - Tip60: updates.
Ghobashi AH, Kamel MA. Ghobashi AH, et al. J Appl Genet. 2018 May;59(2):161-168. doi: 10.1007/s13353-018-0432-y. Epub 2018 Mar 16. J Appl Genet. 2018. PMID: 29549519 Review. - EGFR-Phosphorylated Platelet Isoform of Phosphofructokinase 1 Promotes PI3K Activation.
Lee JH, Liu R, Li J, Wang Y, Tan L, Li XJ, Qian X, Zhang C, Xia Y, Xu D, Guo W, Ding Z, Du L, Zheng Y, Chen Q, Lorenzi PL, Mills GB, Jiang T, Lu Z. Lee JH, et al. Mol Cell. 2018 Apr 19;70(2):197-210.e7. doi: 10.1016/j.molcel.2018.03.018. Mol Cell. 2018. PMID: 29677490 Free PMC article.
References
- Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol. Cell. 2007;28:739–745. - PubMed
- Miller KM, Jackson SP. Histone marks: repairing DNA breaks within the context of chromatin. Biochem. Soc. Trans. 2012;40:370–376. - PubMed
- Downs JA, Nussenzweig MC, Nussenzweig A. Chromatin dynamics and the preservation of genetic information. Nature. 2007;447:951–958. - PubMed
- Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007;19:238–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 092096/WT_/Wellcome Trust/United Kingdom
- C6/A11224/CRUK_/Cancer Research UK/United Kingdom
- A11224/CRUK_/Cancer Research UK/United Kingdom
- 268536/ERC_/European Research Council/International
- 11224/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous