Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo - PubMed (original) (raw)
. 2013 Jun 20;498(7454):371-5.
doi: 10.1038/nature12175. Epub 2013 May 26.
Affiliations
- PMID: 23708969
- PMCID: PMC3879961
- DOI: 10.1038/nature12175
Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo
Tim Lämmermann et al. Nature. 2013.
Abstract
Neutrophil recruitment from blood to extravascular sites of sterile or infectious tissue damage is a hallmark of early innate immune responses, and the molecular events leading to cell exit from the bloodstream have been well defined. Once outside the vessel, individual neutrophils often show extremely coordinated chemotaxis and cluster formation reminiscent of the swarming behaviour of insects. The molecular players that direct this response at the single-cell and population levels within the complexity of an inflamed tissue are unknown. Using two-photon intravital microscopy in mouse models of sterile injury and infection, we show a critical role for intercellular signal relay among neutrophils mediated by the lipid leukotriene B4, which acutely amplifies local cell death signals to enhance the radius of highly directed interstitial neutrophil recruitment. Integrin receptors are dispensable for long-distance migration, but have a previously unappreciated role in maintaining dense cellular clusters when congregating neutrophils rearrange the collagenous fibre network of the dermis to form a collagen-free zone at the wound centre. In this newly formed environment, integrins, in concert with neutrophil-derived leukotriene B4 and other chemoattractants, promote local neutrophil interaction while forming a tight wound seal. This wound seal has borders that cease to grow in kinetic concert with late recruitment of monocytes and macrophages at the edge of the displaced collagen fibres. Together, these data provide an initial molecular map of the factors that contribute to neutrophil swarming in the extravascular space of a damaged tissue. They reveal how local events are propagated over large-range distances, and how auto-signalling produces coordinated, self-organized neutrophil-swarming behaviour that isolates the wound or infectious site from surrounding viable tissue.
Figures
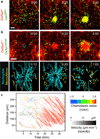
Figure 1. Neutrophil extravascular swarming dynamics
2P-IVM on intact ear dermis of anesthetized mice. Interstitial cell recruitment towards focal damage (blue dotted circle) was recorded. a, b, Time-lapse sequence of endogenous innate immune cell dynamics in DsRed+/−Lyz2 gfp/+Tyrc-2J/c-2J mice (myelomonocytic cells in green-yellow, stroma in red) (a) and DsRed+/+Cx3cr1gfp/gfp Tyrc-2J/c-2J mice (macrophages/monocytes in green, neutrophils and stroma in red). Cell tracks over the last 10 min (n=4) (b, bottom). Scale bars, 50 µm. Time, h:min. c, Distance-time plot (DTP) of intradermal (i.d.) injected bone marrow neutrophils; individual cell-migration paths towards the damage site are each highlighted with instantaneous chemotactic index (colour) and velocity (opacity). Representative experiment of n=169.
Figure 2. LTB4 promotes neutrophil recruitment from distant sites
a, 2P-IVM images of a single neutrophil becoming propidium iodide-positive (arrow) at 13 min and its correlation to neutrophil-amplified chemotaxis (white tracks). DTP analysis for migration paths coloured as in Fig. 1. b, Comparative analysis of interstitial recruitment after i.d. co-injection of _Ltb4r1_−/− and wild-type (WT) neutrophils into Tyrc-2J/c-2J mice. DTP of one representative experiment (n=8). c, Time-course of migration tracks towards 10 µm-damage. Time, h:min. Scale bars, 20 µm (a), 50 µm (c). Track durations, 5 min (a), 10 min (c). d, Comparative analysis of interstitial recruitment after i.d. co-injection of _Ltb4r1_−/− knockout (KO) and wild-type neutrophils into _Alox5_−/− Tyrc-2J/c-2J mice. Radial velocity-time plots with regression lines showing the recruitment dynamics for three individual experiments (of n=7). n (in graph) indicates the number of analyzed tracks. Each dot represents the instantaneous radial velocity for one cell at that time point.
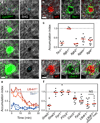
Figure 3. Integrin and GPCR signaling at the neutrophil cluster
a, After focal damage (blue dotted circle), congregating neutrophils rearrange collagenous fibers (visualized by collagen second harmonic generation, SHG). Time, h:min. Scale bar, 50 µm. b–f, Comparative analysis of neutrophil clustering after i.d. co-injection of gene-deficient and wild-type neutrophils into Tyrc-2J/c-2J or _Alox5_−/− Tyrc-2J/c-2J mice. b, d, 2P-IVM images at the endpoint of the clustering response. Scale bars, 50 µm. c, f, Accumulation index as quantitative parameter for neutrophil entry into the collagen-free wound center. Each dot represents analysis of one damage site. Median in red. ns, non-significant (Mann Whitney _U_-test). (e) Time-course of neutrophil accumulation in the wound center. Three representative experiments are presented for each gene-deficiency.
Figure 4. LTB4 requirement for swarming in infected lymph nodes
Mice were infected with _P. aeruginosa_-GFP in the footpad of mice and 2P-IVM was then performed on the draining popliteal lymph nodes at the time indicated. a, Time-lapse sequence of infected subcapsular sinuses of wild-type Lyz2 gfp/+ (top) and Ltb4r1_−/−_Lyz2 gfp/+ (bottom) mice. Analysis was performed when comparable neutrophil numbers were in the sinus (wild-type: 3 h, _Ltb4r1_−/−: 4.5 h). Neutrophil-GFP signal is pseudo-coloured (heat map) to indicate neutrophil clusters (white). Time, h:min. Scale bars, 100 µm. b, c, Quantification of cluster diameter (b) and persistence (c), data pooled from three independent experiments. b, ***: P<0.001 (Student’s _t_-test), c, **: P<0.01 (Mann-Whitney _U_-test). Red bars, mean (b), median (c).
Similar articles
- LTB4 is a signal-relay molecule during neutrophil chemotaxis.
Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA. Afonso PV, et al. Dev Cell. 2012 May 15;22(5):1079-91. doi: 10.1016/j.devcel.2012.02.003. Epub 2012 Apr 26. Dev Cell. 2012. PMID: 22542839 Free PMC article. - Neutrophils self-limit swarming to contain bacterial growth in vivo.
Kienle K, Glaser KM, Eickhoff S, Mihlan M, Knöpper K, Reátegui E, Epple MW, Gunzer M, Baumeister R, Tarrant TK, Germain RN, Irimia D, Kastenmüller W, Lämmermann T. Kienle K, et al. Science. 2021 Jun 18;372(6548):eabe7729. doi: 10.1126/science.abe7729. Science. 2021. PMID: 34140358 Free PMC article. - Neutrophil Swarming in Damaged Tissue Is Orchestrated by Connexins and Cooperative Calcium Alarm Signals.
Poplimont H, Georgantzoglou A, Boulch M, Walker HA, Coombs C, Papaleonidopoulou F, Sarris M. Poplimont H, et al. Curr Biol. 2020 Jul 20;30(14):2761-2776.e7. doi: 10.1016/j.cub.2020.05.030. Epub 2020 Jun 4. Curr Biol. 2020. PMID: 32502410 Free PMC article. - In the eye of the neutrophil swarm-navigation signals that bring neutrophils together in inflamed and infected tissues.
Lämmermann T. Lämmermann T. J Leukoc Biol. 2016 Jul;100(1):55-63. doi: 10.1189/jlb.1MR0915-403. Epub 2015 Sep 28. J Leukoc Biol. 2016. PMID: 26416718 Review. - Neutrophil swarming: an essential process of the neutrophil tissue response.
Kienle K, Lämmermann T. Kienle K, et al. Immunol Rev. 2016 Sep;273(1):76-93. doi: 10.1111/imr.12458. Immunol Rev. 2016. PMID: 27558329 Review.
Cited by
- Dietary habit and risk of rheumatoid arthritis: a mendelian randomization study identifying protective and risk factors.
Li J, Xie X, Chen X, Xie L, Luo M, Yin M, Liu Y, Huang W, Ai Y, He J. Li J, et al. Eur J Nutr. 2024 Nov 13;64(1):3. doi: 10.1007/s00394-024-03518-4. Eur J Nutr. 2024. PMID: 39535565 - Neutrophils under the microscope: neutrophil dynamics in infection, inflammation, and cancer revealed using intravital imaging.
Yam AO, Jakovija A, Gatt C, Chtanova T. Yam AO, et al. Front Immunol. 2024 Oct 8;15:1458035. doi: 10.3389/fimmu.2024.1458035. eCollection 2024. Front Immunol. 2024. PMID: 39439807 Free PMC article. Review. - GATA1-deficient human pluripotent stem cells generate neutrophils with improved antifungal immunity that is mediated by the integrin CD18.
Wagner AS, Smith FM, Bennin DA, Votava JA, Datta R, Giese MA, Zhao W, Skala MC, Fan J, Keller NP, Huttenlocher A. Wagner AS, et al. bioRxiv [Preprint]. 2024 Oct 11:2024.10.11.617742. doi: 10.1101/2024.10.11.617742. bioRxiv. 2024. PMID: 39416161 Free PMC article. Preprint. - Optimized intravital three-photon imaging of intact mouse tibia links plasma cell motility to functional states.
Rakhymzhan A, Fiedler AF, Günther R, Domingue SR, Wooldridge L, Leben R, Cao Y, Bias A, Roodselaar J, Köhler R, Ulbricht C, Heidelin J, Andresen V, Beckers I, Haibel A, Duda G, Hauser AE, Niesner RA. Rakhymzhan A, et al. iScience. 2024 Sep 17;27(10):110985. doi: 10.1016/j.isci.2024.110985. eCollection 2024 Oct 18. iScience. 2024. PMID: 39391739 Free PMC article. - Galvanin is an electric-field sensor for directed cell migration.
Belliveau NM, Footer MJ, Platenkamp A, Kim H, Eustis TE, Theriot JA. Belliveau NM, et al. bioRxiv [Preprint]. 2024 Sep 24:2024.09.23.614580. doi: 10.1101/2024.09.23.614580. bioRxiv. 2024. PMID: 39386424 Free PMC article. Preprint.
References
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. - PubMed
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Rev. Immunol. 2007;7:678–689. - PubMed
- Ng LG, et al. Visualizing the neutrophil response to sterile tissue injury in mouse dermis reveals a three-phase cascade of events. J. Invest. Dermatol. 2011;131:2058–2068. - PubMed
ASSOCIATED REFERENCES
- Rudolph U, et al. Ulcerative colitis and adenocarcinoma of the colon in Gαi2-deficient mice. Nature Genet. 1995;10:143–150. - PubMed
- Potocnik AJ, Brakebusch C, Fässler R. Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity. 2000;12:653–663. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases