Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain - PubMed (original) (raw)
Review
Neurotransmitter-mediated control of neurogenesis in the adult vertebrate brain
Daniel A Berg et al. Development. 2013 Jun.
Abstract
It was long thought that no new neurons are added to the adult brain. Similarly, neurotransmitter signaling was primarily associated with communication between differentiated neurons. Both of these ideas have been challenged, and a crosstalk between neurogenesis and neurotransmitter signaling is beginning to emerge. In this Review, we discuss neurotransmitter signaling as it functions at the intersection of stem cell research and regenerative medicine, exploring how it may regulate the formation of new functional neurons and outlining interactions with other signaling pathways. We consider evolutionary and cross-species comparative aspects, and integrate available results in the context of normal physiological versus pathological conditions. We also discuss the potential role of neurotransmitters in brain size regulation and implications for cell replacement therapies.
Keywords: Adult neurogenesis; Homeostasis; Neural stem cell; Neurotransmitter; Regeneration.
Figures
Fig. 1.
Neurotransmitter signaling and lineage. Alternative mechanisms for neurotransmitter-mediated regulation of cell fate. (A) Each neurotransmitter (NT) controls neurogenesis of its cognate subtype. If neural stem cells (NSCs) are multipotent, transmitters should act on amplifying populations and not on the multipotent stem cell. (B) Neurotransmitters control neurogenesis without subtype specificity, regulating proliferation and differentiation of progenitors but with subtype choices being determined by other factors. If NSCs are multipotent, transmitters could act on both NSCs and/or amplifying populations. (C) Each neurotransmitter controls neurogenesis of its cognate subtype. If NSCs have restricted potential, transmitters could act on both NSCs and/or amplifying populations. (D) Neurotransmitters control neurogenesis without subtype specificity. If NSCs have restricted potential, transmitters could act on both NSCs and/or amplifying populations. The different colors indicate different types of neurotransmitters produced by the neurons. Empty large circles, multipotent NSCs; filled small circles, NSCs with restricted fate; ovals, rectangles and triangles indicate amplifying populations.
Fig. 2.
Interactions between neurotransmitters and other signaling systems. Cell fate decisions during neurogenesis such as proliferation, differentiation and survival could be regulated by neurotransmitters directly (1) or indirectly through regulation of soluble factors (2) and their receptors (3). There are also data supporting the idea that growth factors regulate cell fate decisions through the release of neurotransmitters (4).
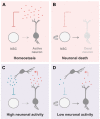
Fig. 3.
Negative control of neurogenesis. (A,B) The neurotransmitter produced directly regulates neurogenesis in a feedback-like manner, as seen in newt midbrain (A). Loss of neurons and consequent drop in neurotransmitter release allows quiescent cells to re-enter the cell cycle (B). (C,D) Neurons regulate neurogenesis through an intermediate neuronal subtype, as seen in the mammalian DG, where GABAergic interneurons inhibit proliferation of stem cells, which give rise to glutamatergic granule neurons. The high activity of the network with high GABA levels counteracts proliferation (C), whereas low activity leads to increased proliferation (D). GABA, γ-aminobutyric acid; NSC, neural stem cell.
Similar articles
- Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis.
Catavero C, Bao H, Song J. Catavero C, et al. Cell Tissue Res. 2018 Jan;371(1):33-46. doi: 10.1007/s00441-017-2668-y. Epub 2017 Sep 25. Cell Tissue Res. 2018. PMID: 28948349 Free PMC article. Review. - A comparative view of regenerative neurogenesis in vertebrates.
Alunni A, Bally-Cuif L. Alunni A, et al. Development. 2016 Mar 1;143(5):741-53. doi: 10.1242/dev.122796. Development. 2016. PMID: 26932669 Free PMC article. Review. - Notch and Neurogenesis.
Engler A, Zhang R, Taylor V. Engler A, et al. Adv Exp Med Biol. 2018;1066:223-234. doi: 10.1007/978-3-319-89512-3_11. Adv Exp Med Biol. 2018. PMID: 30030829 Review. - Locomotion dependent neuron-glia interactions control neurogenesis and regeneration in the adult zebrafish spinal cord.
Chang W, Pedroni A, Bertuzzi M, Kizil C, Simon A, Ampatzis K. Chang W, et al. Nat Commun. 2021 Aug 11;12(1):4857. doi: 10.1038/s41467-021-25052-1. Nat Commun. 2021. PMID: 34381039 Free PMC article. - Nitric oxide regulation of adult neurogenesis.
Gray WP, Cheung A. Gray WP, et al. Vitam Horm. 2014;96:59-77. doi: 10.1016/B978-0-12-800254-4.00004-0. Vitam Horm. 2014. PMID: 25189384 Review.
Cited by
- Going Too Far Is the Same as Falling Short†: Kinesin-3 Family Members in Hereditary Spastic Paraplegia.
Gabrych DR, Lau VZ, Niwa S, Silverman MA. Gabrych DR, et al. Front Cell Neurosci. 2019 Sep 26;13:419. doi: 10.3389/fncel.2019.00419. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31616253 Free PMC article. Review. - Excitatory amino acid transporter 1 supports adult hippocampal neural stem cell self-renewal.
Rieskamp JD, Rosado-Burgos I, Christofi JE, Ansar E, Einstein D, Walters AE, Valentini V, Bruno JP, Kirby ED. Rieskamp JD, et al. iScience. 2023 Jun 8;26(7):107068. doi: 10.1016/j.isci.2023.107068. eCollection 2023 Jul 21. iScience. 2023. PMID: 37534178 Free PMC article. - Adult-born neurons maintain hippocampal cholinergic inputs and support working memory during aging.
Kirshenbaum GS, Chang CY, Bompolaki M, Bradford VR, Bell J, Kosmidis S, Shansky RM, Orlandi J, Savage LM, Harris AZ, David Leonardo E, Dranovsky A. Kirshenbaum GS, et al. Mol Psychiatry. 2023 Dec;28(12):5337-5349. doi: 10.1038/s41380-023-02167-z. Epub 2023 Jul 21. Mol Psychiatry. 2023. PMID: 37479778 - Adult Mammalian Neural Stem Cells and Neurogenesis: Five Decades Later.
Bond AM, Ming GL, Song H. Bond AM, et al. Cell Stem Cell. 2015 Oct 1;17(4):385-95. doi: 10.1016/j.stem.2015.09.003. Cell Stem Cell. 2015. PMID: 26431181 Free PMC article. Review. - Gulf War agent exposure causes impairment of long-term memory formation and neuropathological changes in a mouse model of Gulf War Illness.
Zakirova Z, Tweed M, Crynen G, Reed J, Abdullah L, Nissanka N, Mullan M, Mullan MJ, Mathura V, Crawford F, Ait-Ghezala G. Zakirova Z, et al. PLoS One. 2015 Mar 18;10(3):e0119579. doi: 10.1371/journal.pone.0119579. eCollection 2015. PLoS One. 2015. PMID: 25785457 Free PMC article.
References
- Adolf B., Chapouton P., Lam C. S., Topp S., Tannhäuser B., Strähle U., Götz M., Bally-Cuif L. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295, 278-293 - PubMed
- Agasse F., Bernardino L., Kristiansen H., Christiansen S. H., Ferreira R., Silva B., Grade S., Woldbye D. P., Malva J. O. (2008). Neuropeptide Y promotes neurogenesis in murine subventricular zone. Stem Cells 26, 1636-1645 - PubMed
- Alfonso J., Le Magueresse C., Zuccotti A., Khodosevich K., Monyer H. (2012). Diazepam binding inhibitor promotes progenitor proliferation in the postnatal SVZ by reducing GABA signaling. Cell Stem Cell 10, 76-87 - PubMed
- Andäng M., Hjerling-Leffler J., Moliner A., Lundgren T. K., Castelo-Branco G., Nanou E., Pozas E., Bryja V., Halliez S., Nishimaru H., et al. (2008). Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature 451, 460-464 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources