Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia - PubMed (original) (raw)
Review
Novel roles of reactive oxygen species in the pathogenesis of acute myeloid leukemia
Fuling Zhou et al. J Leukoc Biol. 2013 Sep.
Abstract
It has become apparent that regulation of ROS is important in cell signaling and homeostasis. Accumulation of ROS triggers oxidative stress in various cell types and contributes to the development, progression, and persistence of cancer. Recent research has demonstrated that redox dysregulation caused by ROS promotes proliferation, differentiation, genomic, and epigenetic alterations; immune evasion; and survival in leukemic cells. ROS act as signaling molecules to regulate redox-sensitive transcriptional factors, enzymes, oncogenes, and other downstream effectors. Thus, a thorough understanding the role of ROS as key mediators in leukemogenesis is likely to provide opportunities for improved pharmacological intervention. In this review, we summarize the recent findings that support a role for ROS in the pathogenesis of AML and outline innovative approaches in the implementation of redox therapies for myeloid malignancies.
Keywords: ROS; cell signaling; leukemogenesis.
Figures
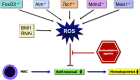
Figure 1.. ROS regulation of hematopoiesis via stem cell self-renewal.
HSCs have self-replicating potential that allows them to constantly replenish blood cells. Elevated ROS levels, present in normal hematopoiesis, lead to reduced self-renewal and hematopoiesis. Elevated ROS levels reduce the self-renewal of HSCs and inhibit hematopoiesis. Deletion of specific genes affects HSC integrity by modulating ROS levels. −/−, Deficient; RNAi, RNA interference.
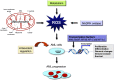
Figure 2.. Altered ROS levels stimulate leukemogenesis.
Oxidative stress has been implicated in the pathogenesis of AML. Aberrant ROS signaling triggers damage to nuclear and mitochondrial DNA and compromises DNA repair via increasing a cell's mutation rate and/or promoting and maintaining the oncogenic phenotype as a secondary messenger in intracellular signaling cascades.
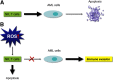
Figure 3.. Immune evasion via ROS.
(A) NK cells are potential effectors of innate immunity that are regulated by efficient immunomodulatory and cytotoxic mechanisms that are essential for eliminating AML cells. (B) Elevated ROS levels trigger apoptosis in NK cells, CD4+ T cells, and CD8+ T cells, whereas AML cells use ROS signaling as a strategy to evade cell-mediated immunity.
Similar articles
- Protein Kinase C Epsilon Is a Key Regulator of Mitochondrial Redox Homeostasis in Acute Myeloid Leukemia.
Di Marcantonio D, Martinez E, Sidoli S, Vadaketh J, Nieborowska-Skorska M, Gupta A, Meadows JM, Ferraro F, Masselli E, Challen GA, Milsom MD, Scholl C, Fröhling S, Balachandran S, Skorski T, Garcia BA, Mirandola P, Gobbi G, Garzon R, Vitale M, Sykes SM. Di Marcantonio D, et al. Clin Cancer Res. 2018 Feb 1;24(3):608-618. doi: 10.1158/1078-0432.CCR-17-2684. Epub 2017 Nov 10. Clin Cancer Res. 2018. PMID: 29127121 Free PMC article. - Reactive oxygen species activate differentiation gene transcription of acute myeloid leukemia cells via the JNK/c-JUN signaling pathway.
Lam CF, Yeung HT, Lam YM, Ng RK. Lam CF, et al. Leuk Res. 2018 May;68:112-119. doi: 10.1016/j.leukres.2018.03.012. Epub 2018 Mar 27. Leuk Res. 2018. PMID: 29609096 - Mitochondrial concept of leukemogenesis: key role of oxygen-peroxide effects.
Lyu BN, Ismailov SB, Ismailov B, Lyu MB. Lyu BN, et al. Theor Biol Med Model. 2008 Nov 11;5:23. doi: 10.1186/1742-4682-5-23. Theor Biol Med Model. 2008. PMID: 19014456 Free PMC article. Review. - Oxidative Stress and ROS-Mediated Signaling in Leukemia: Novel Promising Perspectives to Eradicate Chemoresistant Cells in Myeloid Leukemia.
Trombetti S, Cesaro E, Catapano R, Sessa R, Lo Bianco A, Izzo P, Grosso M. Trombetti S, et al. Int J Mol Sci. 2021 Feb 28;22(5):2470. doi: 10.3390/ijms22052470. Int J Mol Sci. 2021. PMID: 33671113 Free PMC article. Review. - Analysis of the Mechanisms of Action of Naphthoquinone-Based Anti-Acute Myeloid Leukemia Chemotherapeutics.
Lee MH, Lapidus RG, Ferraris D, Emadi A. Lee MH, et al. Molecules. 2019 Aug 28;24(17):3121. doi: 10.3390/molecules24173121. Molecules. 2019. PMID: 31466259 Free PMC article. Review.
Cited by
- Compound kushen injection suppresses human acute myeloid leukaemia by regulating the Prdxs/ROS/Trx1 signalling pathway.
Jin Y, Yang Q, Liang L, Ding L, Liang Y, Zhang D, Wu B, Yang T, Liu H, Huang T, Shen H, Tu H, Pan Y, Wei Y, Yang Y, Zhou F. Jin Y, et al. J Exp Clin Cancer Res. 2018 Nov 19;37(1):277. doi: 10.1186/s13046-018-0948-3. J Exp Clin Cancer Res. 2018. PMID: 30454068 Free PMC article. - Metabolic Plasticity of Acute Myeloid Leukemia.
Kreitz J, Schönfeld C, Seibert M, Stolp V, Alshamleh I, Oellerich T, Steffen B, Schwalbe H, Schnütgen F, Kurrle N, Serve H. Kreitz J, et al. Cells. 2019 Jul 31;8(8):805. doi: 10.3390/cells8080805. Cells. 2019. PMID: 31370337 Free PMC article. Review. - Oxidative stress, redox regulation and diseases of cellular differentiation.
Ye ZW, Zhang J, Townsend DM, Tew KD. Ye ZW, et al. Biochim Biophys Acta. 2015 Aug;1850(8):1607-21. doi: 10.1016/j.bbagen.2014.11.010. Epub 2014 Nov 15. Biochim Biophys Acta. 2015. PMID: 25445706 Free PMC article. Review. - Monocytic Differentiation in Acute Myeloid Leukemia Cells: Diagnostic Criteria, Biological Heterogeneity, Mitochondrial Metabolism, Resistance to and Induction by Targeted Therapies.
Bruserud Ø, Selheim F, Hernandez-Valladares M, Reikvam H. Bruserud Ø, et al. Int J Mol Sci. 2024 Jun 8;25(12):6356. doi: 10.3390/ijms25126356. Int J Mol Sci. 2024. PMID: 38928061 Free PMC article. Review. - Redox-Regulation in Cancer Stem Cells.
Lendeckel U, Wolke C. Lendeckel U, et al. Biomedicines. 2022 Sep 27;10(10):2413. doi: 10.3390/biomedicines10102413. Biomedicines. 2022. PMID: 36289675 Free PMC article. Review.
References
- Finkel T. (2003) Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15, 247–254 - PubMed
- Zhang Y., Du Y., Le W., Wang K., Kieffer N., Zhang J. (2011) Redox control of the survival of healthy and diseased cells. Antioxid. Redox Signal. 15, 2867–2908 - PubMed
- D'Autreaux B., Toledano M. B. (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 - PubMed
- Cairns R. A., Harris I. S., Mak T. W. (2011) Regulation of cancer cell metabolism. Nat. Rev. Cancer 11, 85–95 - PubMed
- Toyokuni S. (2006) Novel aspects of oxidative stress-associated carcinogenesis. Antioxid. Redox Signal. 8, 1373–1377 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical