Perturbation of fetal hematopoiesis in a mouse model of Down syndrome's transient myeloproliferative disorder - PubMed (original) (raw)
Perturbation of fetal hematopoiesis in a mouse model of Down syndrome's transient myeloproliferative disorder
Yehudit Birger et al. Blood. 2013.
Abstract
Children with Down syndrome develop a unique congenital clonal megakaryocytic proliferation disorder (transient myeloproliferative disorder [TMD]). It is caused by an expansion of fetal megakaryocyte-erythroid progenitors (MEPs) triggered by trisomy of chromosome 21 and is further enhanced by the somatic acquisition of a mutation in GATA1. These mutations result in the expression of a short-isoform GATA1s lacking the N-terminal domain. To examine the hypothesis that the Hsa21 ETS transcription factor ERG cooperates with GATA1s in this process, we generated double-transgenic mice expressing hERG and Gata1s. We show that increased expression of ERG by itself is sufficient to induce expansion of MEPs in fetal livers. Gata1s expression synergizes with ERG in enhancing the expansion of fetal MEPs and megakaryocytic precursors, resulting in hepatic fibrosis, transient postnatal thrombocytosis, anemia, a gene expression profile that is similar to that of human TMD and progression to progenitor myeloid leukemia by 3 months of age. This ERG/Gata1s transgenic mouse model also uncovers an essential role for the N terminus of Gata1 in erythropoiesis and the antagonistic role of ERG in fetal erythroid differentiation and survival. The human relevance of this finding is underscored by the recent discovery of similar mutations in GATA1 in patients with Diamond-Blackfan anemia.
Figures
Figure 1
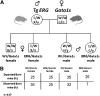
Generation of ERG/Gata1s mice. (A) TgERG males were mated with Gata1s knock-in homozygous females to generate animals that express the hERG transgene and the short isoform of GATA1, GATA1s. As GATA1 is located on the X chromosome; male offspring from this cross are all hemizygous and females are all heterozygous for GATA1s. Half of the animals carry the ERG transgene. (B) A table representing the observed vs the expected born mice from the different genotypes. Statistical significance was tested using the Fisher exact test (P < .01 for ERG/Gata1s males and P = .09 for ERG/Gata1s females; n(total) = 137) and the χ2 test (P = .0047).
Figure 2
Expansion of MEPs in TgERG embryo FLs is enhanced by Gata1s. (A) Representative flow cytometric analysis showing immunostaining of lineage-negative (Lin−) cells. HSCs are c-Kit+ and Sca1+ and HPCs are c-Kit+ and Sca−. HPCs were subclassified into GMPs (FcγR+ and CD34+), CMPs (FcγR− and CD34+), and MEPs (FcγR− and CD34−), respectively. (B) Relative progenitor and stem cell populations in wild-type and TgERG E14.5 FLs. (C) Distribution of myeloid progenitors in wild-type and TgERG E14.5 FLs. (D) Relative progenitor and stem cell populations in Wt/Gata1s and ERG/Gata1s E14.5 FLs. (E) Distribution of myeloid progenitors in Wt/Gata1s and ERG/Gata1s E14.5 FLs. The bar graphs represent the average of at least 3 independent experiments with n >10 embryos for each genotype. Statistical significant differences (t test for pairs and ANOVA for groups) are detailed in the figure. ANOVA, analysis of variance.
Figure 3
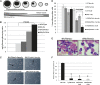
ERG synergizes with Gata1s to promote transient megakaryocytic proliferation presenting a human TMD expression profile. (A-C) FL cells were isolated from E12.5 (A-B) and E14.5 (C) wild-type, TgERG embryos and males and females of Wt/Gata1s and ERG/Gata1s embryos. The cells were plated on methylcellulose supplemented with TPO to promote the growth of megakaryocytic colonies (CFU-MK). MK colonies were counted 5 to 7 days following plating. Each bar graph represents the average of at least 3 independent experiments. (D) Representative figure of megakaryocytic colonies from (top) TgERG, (middle) Wt/Gata1s, and (bottom) ERG/Gata1s FLs. (E) Real-time PCR of common human TMD genes (11). *Statistical significance was tested using the t test (P = .028 for Mpl; P = .009 for Pecam1; and P = .0 for Mycn). (F) Gene set enrichment analysis (GSEA) using gene expression of E14.5 ERG/Gata1s FL cells (GSE46481) compared with the respective Wt control shows significant enrichment of genes that were upregulated in human TMD (GSE4119). NES and FDR q values are shown. (G) Heat map showing the core enrichment genes from the GSEA presented in panel F. (H) Hepatic fibrosis in ERG/Gata1s male FL. Reticulin staining (arrowheads) of (left) Wt/Gata1s and (right) ERG/Gata1s FL tissues (magnification, ×400). (I) Transient thrombocytosis in TgERG and ERG/Gata1s mice. Platelet counts were retrieved from male and female TgERG and ERG/Gata1s and their Wt and Wt/Gata1s littermates at 3 and 7 weeks of age; n= at least 6 for each group. A significant difference was found between TgERG and Wt and between ERG/Gata1s and Wt/Gata1s (t test: P < .017, P < .0031, respectively). FDR, false detection rate; NES, normalized enrichment score.
Figure 4
Requirement of the N terminus of GATA1 in fetal erythropoiesis. (A) Representative pictures of E14.5 embryos. The FL of ERG/Gata1s males is smaller and paler (arrow). A total of 1 × 107 FL cells were resuspended in PBS. The light color of the ERG/Gata1s FL cells points to the decrease number in mature erythrocytes in those embryos. (B) ERG/Gata1s males die between E12.5 and E14.5. Females and males generated from crossing heterozygous TgERG males with homozygous Gata1s KI females were tested for the presence of ERG transgene on E12.5, E14.5, and adults (n = 85, 98, and 137 for E12.5, E14.5, and adults, respectively). A significant difference in the TgERG male population was found between E12.5 and E14.5 (t test: P < .014). (C-E) ERG and Gata1s inhibit erythroid colony formation. FL cells were isolated from (C) E12.5 TgERG embryos and Wt littermates and (D-E) E12.5 and E14.5 embryos of ERG/Gata1s embryos and Wt/Gata1s littermates and were plated on methylcellulose supplemented with EPO to promote growth of erythroid colonies (BFU-E). Erythroid colonies (BFU-E) were counted 7 to 10 days after plating. Each bar graph represents the average of at least 3 independent experiments. Statistical significance was tested using the t test. (F) Representative figures of flow cytometric analysis of male E12.5 FL cells using the erythroid markers Ter119 and CD71. (G) Expression of Gata1s impairs erythropoiesis. Expression of Ter119 erythroid marker as measured by flow cytometry analysis in FL cells generated from E12.5 females and males of Wt, TgERG, Gata1s, and ERG/Gata1s animals. (H) Increase apoptosis in ERG/Gata1s males. Annexin V levels were measured by flow cytometry in Ter119-positive FL cells for the detection of apoptosis. FL cells were isolated from E12.5 Wt, TgERG, Wt/Gata1s, and ERG/Gata1s males. Significant increase in apoptosis in ERG/Gata1s males compared with Wt/Gata1s males was measured using the t test (P = .017). (I) Decreased expression of erythroid and antiapoptotic genes in ERG/Gata1s males. Expression level of the different genes was obtained from the Affymetrix mouse gene 1.0 ST chip array and confirmed by real-time PCR on at least 2 additional samples for each genotype.
Figure 5
Erythroid differentiation arrest in ERG/GATA1s mice. (A) Schematic representation of erythroid differentiation indicating early erythrocytes as Ter119+/CD71+ and late differentiated erythrocytes as Ter119+/CD71−. (B) Increase in early erythroblast population compared with late differentiated erythrocytes in ERG/Gata1s males. FL cells were isolated from E14.5 Wild-type, TgERG, Wt/Gata1s, and ERG/Gata1s male embryos and stained with Ter119 and CD71. Statistical significance between Wt embryos and the remaining genotypes was measured using the t test. (C-D) Reduced benzidine-stained cells in ERG/Gata1s males. Representative images of benzidine-stained FL cells (C) and a graph averaging the ratio of benzidine-positive cells in FLs isolated from E14.5 Wt, TgERG, Wt/Gata1s, and ERG/Gata1s male and female embryos (D). The bar graph represents the average of at least 3 experiments. (E) Giemsa stain of cytospins of E14.5 FL cells show a decrease in maturing erythroblasts and increase in immature proerythroblasts in ERG/Gata1s males compared with Wt/Gata1s males. (F) Expression of hemoglobin. Adult hemoglobin β major chain (Hbb-b1) expression was measured by real-time PCR in E14.5 FL cells from Wt, TgERG, Wt/Gata1s, and ERG/Gata1s males. *Significant difference from Wt hemoglobin expression was measured using the t test (P = .00037 for TgERG; P < .000001 for Wt/Gata1s; and P < .00001 for ERG/Gata1s).
Figure 6
ERG/Gata1s FL cells present an early erythroid expression profile. (A) GSEA using gene expression of human erythroid cells at different stages of differentiation shows enrichment of Wt/Gata1s and ERG/GATA1s E14.5 FL gene signature (GSE46481) in early erythroid cells. (B) Heat map showing the top 20 core enrichment genes from the GSEA presented in panel A, right. (C) Expression level of Gata2 and Myb genes as obtained from the mouse gene 1.0 ST chip array and confirmed by real-time PCR on at least 2 additional samples for each genotype. (D) Representative western blot from G1ME cells transduced with MigR1, MIGR1-GATA1, and MIGR1-GATA1s using the indicated antibodies. (E) Average difference in GATA2 expression from 3 independent G1ME expreriments presented in panel D. (F) An average repression of GATA2 mRNA by real-time PCR in E14.5 GATA1s KI FL cells infected with MIGR1-GATA1and MIGR1-GATA1s relative to MigR1-infected cells. Relative GATA1 and GATA1s RNA expression levels are indicated below (n = 3).
Figure 7
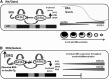
Schematic representation of the main events leading to impaired fetal erythropoiesis. (A) Gata2 which is expressed in erythroid progenitors, positively regulates Gata1 and its own expression. Gata1 also positively regulates its own expression but represses Gata2. Progression in erythroid differentiation becomes possible due to the Gata1/Gata2 switch on the promoter of erythroid genes resulting in their activation. Gata2 repression is an essential step in erythroid differentiation and is executed by several factors including Gata1 and the decline in ERG expression. (B) In the ERG/Gata1s mice, several factors block erythroid differentiation. In the absence of the N-terminal domain of Gata1, Gata1s fails to repress Gata2, erythroid genes are not activated, and differentiation is blocked. Furthermore, the Gata2 repressor Myb is downregulated by ERG and Gata1s. Because Myb functions as a Gata2 repressor, decreased Myb levels result in an increase in Gata2 expression. In the ERG transgenic model, the ERG expression level fails to decline with erythroid differentiation, thus further maintaining Gata2 expression and preventing erythroid cells to fully differentiate to erythrocytes.
Similar articles
- ETS2 and ERG promote megakaryopoiesis and synergize with alterations in GATA-1 to immortalize hematopoietic progenitor cells.
Stankiewicz MJ, Crispino JD. Stankiewicz MJ, et al. Blood. 2009 Apr 2;113(14):3337-47. doi: 10.1182/blood-2008-08-174813. Epub 2009 Jan 23. Blood. 2009. PMID: 19168790 Free PMC article. - Early lineage priming by trisomy of Erg leads to myeloproliferation in a Down syndrome model.
Ng AP, Hu Y, Metcalf D, Hyland CD, Ierino H, Phipson B, Wu D, Baldwin TM, Kauppi M, Kiu H, Di Rago L, Hilton DJ, Smyth GK, Alexander WS. Ng AP, et al. PLoS Genet. 2015 May 14;11(5):e1005211. doi: 10.1371/journal.pgen.1005211. eCollection 2015 May. PLoS Genet. 2015. PMID: 25973911 Free PMC article. - Pluripotent stem cell model of early hematopoiesis in Down syndrome reveals quantitative effects of short-form GATA1 protein on lineage specification.
Matsuo S, Nishinaka-Arai Y, Kazuki Y, Oshimura M, Nakahata T, Niwa A, Saito MK. Matsuo S, et al. PLoS One. 2021 Mar 29;16(3):e0247595. doi: 10.1371/journal.pone.0247595. eCollection 2021. PLoS One. 2021. PMID: 33780474 Free PMC article. - Molecular insights into Down syndrome-associated leukemia.
Vyas P, Crispino JD. Vyas P, et al. Curr Opin Pediatr. 2007 Feb;19(1):9-14. doi: 10.1097/MOP.0b013e328013e7b2. Curr Opin Pediatr. 2007. PMID: 17224656 Review. - GATA1 in Normal and Pathologic Megakaryopoiesis and Platelet Development.
Takasaki K, Chou ST. Takasaki K, et al. Adv Exp Med Biol. 2024;1459:261-287. doi: 10.1007/978-3-031-62731-6_12. Adv Exp Med Biol. 2024. PMID: 39017848 Review.
Cited by
- Down syndrome-associated leukaemias: current evidence and challenges.
Mason NR, Cahill H, Diamond Y, McCleary K, Kotecha RS, Marshall GM, Mateos MK. Mason NR, et al. Ther Adv Hematol. 2024 Jul 23;15:20406207241257901. doi: 10.1177/20406207241257901. eCollection 2024. Ther Adv Hematol. 2024. PMID: 39050114 Free PMC article. Review. - Synergistic roles of DYRK1A and GATA1 in trisomy 21 megakaryopoiesis.
Sit YT, Takasaki K, An HH, Xiao Y, Hurtz C, Gearhart PA, Zhang Z, Gadue P, French DL, Chou ST. Sit YT, et al. JCI Insight. 2023 Oct 31;8(23):e172851. doi: 10.1172/jci.insight.172851. JCI Insight. 2023. PMID: 37906251 Free PMC article. - The NCOR-HDAC3 co-repressive complex modulates the leukemogenic potential of the transcription factor ERG.
Kugler E, Madiwale S, Yong D, Thoms JAI, Birger Y, Sykes DB, Schmoellerl J, Drakul A, Priebe V, Yassin M, Aqaqe N, Rein A, Fishman H, Geron I, Chen CW, Raught B, Liu Q, Ogana H, Liedke E, Bourquin JP, Zuber J, Milyavsky M, Pimanda J, Privé GG, Izraeli S. Kugler E, et al. Nat Commun. 2023 Sep 21;14(1):5871. doi: 10.1038/s41467-023-41067-2. Nat Commun. 2023. PMID: 37735473 Free PMC article. - RUNX1 isoform disequilibrium promotes the development of trisomy 21-associated myeloid leukemia.
Gialesaki S, Bräuer-Hartmann D, Issa H, Bhayadia R, Alejo-Valle O, Verboon L, Schmell AL, Laszig S, Regényi E, Schuschel K, Labuhn M, Ng M, Winkler R, Ihling C, Sinz A, Glaß M, Hüttelmaier S, Matzk S, Schmid L, Strüwe FJ, Kadel SK, Reinhardt D, Yaspo ML, Heckl D, Klusmann JH. Gialesaki S, et al. Blood. 2023 Mar 9;141(10):1105-1118. doi: 10.1182/blood.2022017619. Blood. 2023. PMID: 36493345 Free PMC article. - Leukemogenesis in infants and young children with trisomy 21.
Roberts I. Roberts I. Hematology Am Soc Hematol Educ Program. 2022 Dec 9;2022(1):1-8. doi: 10.1182/hematology.2022000395. Hematology Am Soc Hematol Educ Program. 2022. PMID: 36485097 Free PMC article.
References
- Henry E, Walker D, Wiedmeier SE, Christensen RD. Hematological abnormalities during the first week of life among neonates with Down syndrome: data from a multihospital healthcare system. Am J Med Genet A. 2007;143(1):42–50. - PubMed
- Mundschau G, Gurbuxani S, Gamis AS, Greene ME, Arceci RJ, Crispino JD. Mutagenesis of GATA1 is an initiating event in Down syndrome leukemogenesis. Blood. 2003;101(11):4298–4300. - PubMed
- Pine SR, Guo Q, Yin C, Jayabose S, Druschel CM, Sandoval C. Incidence and clinical implications of GATA1 mutations in newborns with Down syndrome. Blood. 2007;110(6):2128–2131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases