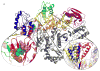The interplay of structure and dynamics: insights from a survey of HIV-1 reverse transcriptase crystal structures - PubMed (original) (raw)
. 2013 Oct;81(10):1792-801.
doi: 10.1002/prot.24325. Epub 2013 Aug 16.
Affiliations
- PMID: 23720322
- PMCID: PMC3773008
- DOI: 10.1002/prot.24325
The interplay of structure and dynamics: insights from a survey of HIV-1 reverse transcriptase crystal structures
James M Seckler et al. Proteins. 2013 Oct.
Abstract
HIV-1 reverse transcriptase (RT) is a critical drug target for HIV treatment, and understanding the exact mechanisms of its function and inhibition would significantly accelerate the development of new anti-HIV drugs. It is well known that structure plays a critical role in protein function, but for RT, structural information has proven to be insufficient-despite enormous effort-to explain the mechanism of inhibition and drug resistance of non-nucleoside RT inhibitors. We hypothesize that the missing link is dynamics, information about the motions of the system. However, many of the techniques that give the best information about dynamics, such as solution nuclear magnetic resonance and molecular dynamics simulations, cannot be easily applied to a protein as large as RT. As an alternative, we combine elastic network modeling with simultaneous hierarchical clustering of structural and dynamic data. We present an extensive survey of the dynamics of RT bound to a variety of ligands and with a number of mutations, revealing a novel mechanism for drug resistance to non-nucleoside RT inhibitors. Hydrophobic core mutations restore active-state motion to multiple functionally significant regions of HIV-1 RT. This model arises out of a combination of structural and dynamic information, rather than exclusively from one or the other.
Keywords: allostery; elastic network model; protein-drug interactions; reverse transcriptase inhibition; structure-function.
Copyright © 2013 Wiley Periodicals, Inc.
Figures
Figure 1
The structure of HIV-1 RT containing the larger subunit (p66) has a polymerase domain consisting of a fingers (blue), palm (red), thumb (green), and connection (orange) subdomain and an RNase H (purple) domain. The smaller subunit (p51) has the same N-terminal sequence as p66 (gray), but lacks the RNase H domain. (b) The NNRTI binding pocket with the NNRTI (cyan, spheres) and drug resistant mutants shown in spheres colored by if they are hydrophobic core mutations (purple) or entry blocker mutations (orange). (c) The change in the position of the thumb subdomain depending on which ligand RT is bound to: unliganded (red; 1DLO), DNA-bound (blue; 1N5Y), or NNRTI bound (yellow; 1VRT) .
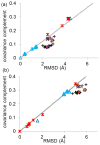
Figure 2
The covariance complement and RMSD of all 52 structures compared to (a) wild type RT bound to DNA (1N6Q), and (b) unliganded RT (1DLO). Points are shaped and colored by the ligand and mutations: NNRTI (black pluses), RT bound to DNA (light blue triangles), RNA (dark blue triangle), unliganded (red stars), entry blocker mutants bound to susceptible NNRTI (orange Xs), and hydrophobic core mutants bound to susceptible NNRTI (purple Xs). The best fit line to either all DNA-bound RT (a) or unliganded RT (b) is shown in gray. Structures that show a linear relationship between RMSD and covariance complement tend to show similar functional abilities, while proteins which form off diagonal clusters tend to have different functional abilities. This is true even for very different structures (DNA-bound and unliganded) ; .
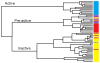
Figure 3
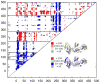
Agglomerative hierarchical clustering was employed on the ratio of covariance complement to RMSD, forming an active, pre-active, and inactive cluster. The resulting clusters are colored by their ligand: NNRTI-bound drug inhibited mutants (black), 1st/2nd Generation NNRTI-bound hydrophobic core mutants (purple), 1st/2nd Generation NNRTI-bound entry blocker mutants (orange), DNA (light blue), RNA (dark blue), and unliganded (red). The sole structure bound to both DNA and NNRTIs is striped black and light blue.
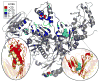
Figure 4
The structure of RT colored by the difference in contacts in the connectivity matrix (Γ_ij_) between the pre-active to the inactive cluster. (b) shows the shift in the thumbs position between two structures in the NNRTI-bound pre-active cluster (red) and two structures from the NNRTI inactive cluster (yellow). The thumb subdomain rotates away from the connection subdomain. (c) shows a the subtle rotation in β-12-13-14 which forms half of the drug binding pocket ; ; ; .
Figure 5
We calculated a matrix of significant differences between the inter-residue correlations of the inactive and pre-active cluster according to the Welch’s T-test. Residue pairs showing a significant difference between the pre-active and inactive cluster are colored. This difference can be more correlated (red) or more anti-correlated (blue) fashion. The residues showing a strong difference in their inter-residue correlation tend to be regions of the protein which begin moving differently with regards to the rest of the structure. The number of residues changing their motion with respect to a single residue mapped onto the structure of p66. The structure is colored by whether residues become more correlated moving from the inactive to the pre-active cluster (yellow, orange, red), or more anti-correlated (blue, cyan, purple) .
Similar articles
- Structural mechanisms of drug resistance for mutations at codons 181 and 188 in HIV-1 reverse transcriptase and the improved resilience of second generation non-nucleoside inhibitors.
Ren J, Nichols C, Bird L, Chamberlain P, Weaver K, Short S, Stuart DI, Stammers DK. Ren J, et al. J Mol Biol. 2001 Sep 28;312(4):795-805. doi: 10.1006/jmbi.2001.4988. J Mol Biol. 2001. PMID: 11575933 - Evolving understanding of HIV-1 reverse transcriptase structure, function, inhibition, and resistance.
Xavier Ruiz F, Arnold E. Xavier Ruiz F, et al. Curr Opin Struct Biol. 2020 Apr;61:113-123. doi: 10.1016/j.sbi.2019.11.011. Epub 2020 Jan 11. Curr Opin Struct Biol. 2020. PMID: 31935541 Free PMC article. Review. - Crystal structures of HIV-1 reverse transcriptases mutated at codons 100, 106 and 108 and mechanisms of resistance to non-nucleoside inhibitors.
Ren J, Nichols CE, Chamberlain PP, Weaver KL, Short SA, Stammers DK. Ren J, et al. J Mol Biol. 2004 Feb 20;336(3):569-78. doi: 10.1016/j.jmb.2003.12.055. J Mol Biol. 2004. PMID: 15095972 - The Lys103Asn mutation of HIV-1 RT: a novel mechanism of drug resistance.
Hsiou Y, Ding J, Das K, Clark AD Jr, Boyer PL, Lewi P, Janssen PA, Kleim JP, Rösner M, Hughes SH, Arnold E. Hsiou Y, et al. J Mol Biol. 2001 Jun 1;309(2):437-45. doi: 10.1006/jmbi.2001.4648. J Mol Biol. 2001. PMID: 11371163 - HIV-1 reverse transcriptase and antiviral drug resistance. Part 2.
Das K, Arnold E. Das K, et al. Curr Opin Virol. 2013 Apr;3(2):119-28. doi: 10.1016/j.coviro.2013.03.014. Epub 2013 Apr 19. Curr Opin Virol. 2013. PMID: 23602470 Free PMC article. Review.
Cited by
- Conformational Plasticity of the NNRTI-Binding Pocket in HIV-1 Reverse Transcriptase: A Fluorine Nuclear Magnetic Resonance Study.
Sharaf NG, Ishima R, Gronenborn AM. Sharaf NG, et al. Biochemistry. 2016 Jul 19;55(28):3864-73. doi: 10.1021/acs.biochem.6b00113. Epub 2016 Jul 11. Biochemistry. 2016. PMID: 27163463 Free PMC article. - Structure-Encoded Global Motions and Their Role in Mediating Protein-Substrate Interactions.
Bahar I, Cheng MH, Lee JY, Kaya C, Zhang S. Bahar I, et al. Biophys J. 2015 Sep 15;109(6):1101-9. doi: 10.1016/j.bpj.2015.06.004. Epub 2015 Jul 2. Biophys J. 2015. PMID: 26143655 Free PMC article. Review. - Enzymatic Transition States and Drug Design.
Schramm VL. Schramm VL. Chem Rev. 2018 Nov 28;118(22):11194-11258. doi: 10.1021/acs.chemrev.8b00369. Epub 2018 Oct 18. Chem Rev. 2018. PMID: 30335982 Free PMC article. Review. - Structure-based simulations reveal concerted dynamics of GPCR activation.
Leioatts N, Suresh P, Romo TD, Grossfield A. Leioatts N, et al. Proteins. 2014 Oct;82(10):2538-51. doi: 10.1002/prot.24617. Epub 2014 Jun 9. Proteins. 2014. PMID: 24889093 Free PMC article. - Small Conformational Changes Underlie Evolution of Resistance to NNRTI in HIV Reverse Transcriptase.
Srivastava A, Birari V, Sinha S. Srivastava A, et al. Biophys J. 2020 May 19;118(10):2489-2501. doi: 10.1016/j.bpj.2020.04.008. Epub 2020 Apr 16. Biophys J. 2020. PMID: 32348721 Free PMC article.
References
- Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–90. - PubMed
- Boyer PL, Ferris AL, Clark P, Whitmer J, Frank P, Tantillo C, Arnold E, Hughes SH. Mutational analysis of the fingers and palm subdomains of human immunodeficiency virus type-1 (HIV-1) reverse transcriptase. J Mol Biol. 1994;243:472–83. - PubMed
- Bahar I, Erman B, Jernigan RL, Atilgan AR, Covell DG. Collective motions in HIV-1 reverse transcriptase: examination of flexibility and enzyme function. J Mol Biol. 1999;285:1023–37. - PubMed
- Perno CF, Yarchoan R, Cooney DA, Hartman NR, Gartner S, Popovic M, Hao Z, Gerrard TL, Wilson YA, Johns DG, et al. Inhibition of human immunodeficiency virus (HIV-1/HTLV-IIIBa-L) replication in fresh and cultured human peripheral blood monocytes/macrophages by azidothymidine and related 2′,3′-dideoxynucleosides. J Exp Med. 1988;168:1111–25. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI083206/AI/NIAID NIH HHS/United States
- T32AI083206/AI/NIAID NIH HHS/United States
- 272201000055C/PHS HHS/United States
- GM068411/GM/NIGMS NIH HHS/United States
- R01 GM095496/GM/NIGMS NIH HHS/United States
- T32 GM068411/GM/NIGMS NIH HHS/United States
- P30 AI078498/AI/NIAID NIH HHS/United States
- GM095496/GM/NIGMS NIH HHS/United States
- HHSN272201000055C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials