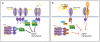The Drosophila IMD pathway in the activation of the humoral immune response - PubMed (original) (raw)
Review
The Drosophila IMD pathway in the activation of the humoral immune response
Anni Kleino et al. Dev Comp Immunol. 2014 Jan.
Abstract
The IMD pathway signaling plays a pivotal role in the Drosophila defense against bacteria. During the last two decades, significant progress has been made in identifying the components and deciphering the molecular mechanisms underlying this pathway, including the means of bacterial sensing and signal transduction. While these findings have contributed to the understanding of the immune signaling in insects, they have also provided new insights in studying the mammalian NF-κB signaling pathways. Here, we summarize the current view of the IMD pathway focusing on how it regulates the humoral immune response of Drosophila.
Keywords: 20; 20-hydroxyecdysone; AMP; DAP; DREDD; Drosophila; FADD; Fas-associated protein with Death Domain; IAP; IKK; Immune response; IκB; IκB kinase; JNK; NF-κB signaling; PGN; PGRP; RHIM; RIP; RIP homotypic interaction motif; RNA interference; RNAi; TAB; TAK1; TAK1-associated binding protein 2; TCT; TGF-β activated kinase 1; antimicrobial peptide; c-Jun N-terminal kinase; death-related ced-3/Nedd2-like protein; imd; immune deficiency; inhibitor of apoptosis; inhibitor of κB; meso-diaminopimelic acid; peptidoglycan; peptidoglycan recognition protein; receptor-interacting protein; tracheal cytotoxin.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
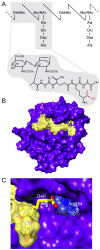
Figure 1
The principle of DAP-PGN recognition by PGRP-LE. (A) Schematic illustration of polymeric DAP-PGN and TCT. The carboxylate group of DAP that interacts with Arg254 of PGRP-LE is highlighted with red color. (B) Structure of the PGRP domain of PGRP-LE bound to TCT. (C) Atomic-level interactions of Arg254 from PGRP-LE and the carboxylic acid specific to DAP from TCT.
Figure 2
Positive and negative regulation of the receptor-proximal signaling events in the IMD pathway. (A) Activation of the IMD pathway signaling by polymeric and monomeric DAP-PGN through recognition by PGRP-LC on the plasma membrane, or PGRP-LE in the cytoplasm. TCT likely multimerizes intracellular PGRP-LE, dimerizes cell surface PGRP-LCx and –LCa, while polymeric DAP-type peptidoglycan is thought to cluster PGRP-LCx. Ligand binding is believed to lead to the recruitment of IMD, dFADD, and the caspase-8 homolog DREDD to the signaling complex. (B) Negative regulation of receptor activation. Active amidases PGRP-LB and PGRP-SC digest polymeric PGN to small subunits that do not activate PGRP-LC. PGRP-LF binds PGRP-LC and blocks signaling by competitive inhibition. PIRK binds both IMD and the RHIM-like motif found in PGRP-LC and PGRP-LE, which suppresses the receptor-IMD interaction and downstream signaling events.
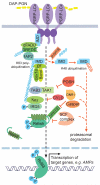
Figure 3
Ubiquitin signaling in the activation and deactivation of the IMD pathway signaling. Following PGN recognition and the assembly of a receptor proximal signaling complex, IMD signaling proceeds through at least two distinct paths. Both paths require the proteolytic activity of DREDD, which presumably acts as part of this receptor proximal complex. Once activated, DREDD cleaves Relish, which is necessary for its activation, nuclear translocation and induction of target genes. DREDD also likely cleaves IMD, exposing an IBM at position 31. Cleaved IMD then interacts with IAP2 and is robustly and transiently conjugated with K63-polyubiquitin chains. These chains are thought to serve as a scaffold for both the TAK1/TAB2 complex, through NZF domain of TAB2, and the IKK complex, through the IKKγ subunit. The IKK complex then phosphorylates Relish, which is important for its activation, and contributes in a non-catalytic manner to Relish cleavage. The IMD pathway is also subject to numerous negative regulatory controls, including deubiquitination of IMD by dUSP36. IMD may then be re-ubiquitinated with K48-chains and degraded. Likewise, the ubiquitin-proteasome system is implicated in the control of TAK1, DREDD and Relish by POSH, DNR1 and the SCF complex respectively.
Similar articles
- Peptidoglycan recognition proteins in Drosophila immunity.
Kurata S. Kurata S. Dev Comp Immunol. 2014 Jan;42(1):36-41. doi: 10.1016/j.dci.2013.06.006. Epub 2013 Jun 22. Dev Comp Immunol. 2014. PMID: 23796791 Free PMC article. Review. - Peptidoglycan-Sensing Receptors Trigger the Formation of Functional Amyloids of the Adaptor Protein Imd to Initiate Drosophila NF-κB Signaling.
Kleino A, Ramia NF, Bozkurt G, Shen Y, Nailwal H, Huang J, Napetschnig J, Gangloff M, Chan FK, Wu H, Li J, Silverman N. Kleino A, et al. Immunity. 2017 Oct 17;47(4):635-647.e6. doi: 10.1016/j.immuni.2017.09.011. Immunity. 2017. PMID: 29045898 Free PMC article. - PGRP-SD, an Extracellular Pattern-Recognition Receptor, Enhances Peptidoglycan-Mediated Activation of the Drosophila Imd Pathway.
Iatsenko I, Kondo S, Mengin-Lecreulx D, Lemaitre B. Iatsenko I, et al. Immunity. 2016 Nov 15;45(5):1013-1023. doi: 10.1016/j.immuni.2016.10.029. Immunity. 2016. PMID: 27851910 - Innate immune signaling in Drosophila is regulated by transforming growth factor β (TGFβ)-activated kinase (Tak1)-triggered ubiquitin editing.
Chen L, Paquette N, Mamoor S, Rus F, Nandy A, Leszyk J, Shaffer SA, Silverman N. Chen L, et al. J Biol Chem. 2017 May 26;292(21):8738-8749. doi: 10.1074/jbc.M117.788158. Epub 2017 Apr 4. J Biol Chem. 2017. PMID: 28377500 Free PMC article. - Functional genomic analysis of the Drosophila immune response.
Valanne S. Valanne S. Dev Comp Immunol. 2014 Jan;42(1):93-101. doi: 10.1016/j.dci.2013.05.007. Epub 2013 May 21. Dev Comp Immunol. 2014. PMID: 23707784 Review.
Cited by
- Inflammation-Induced, STING-Dependent Autophagy Restricts Zika Virus Infection in the Drosophila Brain.
Liu Y, Gordesky-Gold B, Leney-Greene M, Weinbren NL, Tudor M, Cherry S. Liu Y, et al. Cell Host Microbe. 2018 Jul 11;24(1):57-68.e3. doi: 10.1016/j.chom.2018.05.022. Epub 2018 Jun 19. Cell Host Microbe. 2018. PMID: 29934091 Free PMC article. - Transcript analysis reveals the involvement of NF-κB transcription factors for the activation of TGF-β signaling in nematode-infected Drosophila.
Patrnogic J, Heryanto C, Ozakman Y, Eleftherianos I. Patrnogic J, et al. Immunogenetics. 2019 Jul;71(7):501-510. doi: 10.1007/s00251-019-01119-8. Epub 2019 May 30. Immunogenetics. 2019. PMID: 31147740 - Transcriptomic Analysis Reveals the Impact of the Biopesticide Metarhizium anisopliae on the Immune System of Major Workers in Solenopsis invicta.
Wu H, Xu Y, Zafar J, Mandal S, Lin L, Lu Y, Jin F, Pang R, Xu X. Wu H, et al. Insects. 2023 Aug 11;14(8):701. doi: 10.3390/insects14080701. Insects. 2023. PMID: 37623411 Free PMC article. - Insulin-Like Peptides and Cross-Talk With Other Factors in the Regulation of Insect Metabolism.
Chowański S, Walkowiak-Nowicka K, Winkiel M, Marciniak P, Urbański A, Pacholska-Bogalska J. Chowański S, et al. Front Physiol. 2021 Jun 29;12:701203. doi: 10.3389/fphys.2021.701203. eCollection 2021. Front Physiol. 2021. PMID: 34267679 Free PMC article. Review. - Control of Drosophila blood cell activation via Toll signaling in the fat body.
Schmid MR, Anderl I, Vesala L, Vanha-aho LM, Deng XJ, Rämet M, Hultmark D. Schmid MR, et al. PLoS One. 2014 Aug 7;9(8):e102568. doi: 10.1371/journal.pone.0102568. eCollection 2014. PLoS One. 2014. PMID: 25102059 Free PMC article.
References
- Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 AI060025/AI/NIAID NIH HHS/United States
- AI099708/AI/NIAID NIH HHS/United States
- AI060025/AI/NIAID NIH HHS/United States
- R01 AI074958/AI/NIAID NIH HHS/United States
- R01 AI099708/AI/NIAID NIH HHS/United States
- R01 AI060025/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous