Retromer-mediated endosomal protein sorting: all WASHed up! - PubMed (original) (raw)
Review
Retromer-mediated endosomal protein sorting: all WASHed up!
Matthew N J Seaman et al. Trends Cell Biol. 2013 Nov.
Abstract
Endosomal protein sorting governs the fate of many physiologically important proteins involved in a panoply of cellular functions. Recent discoveries have revealed a vital role for endosomally localised branched actin patches in facilitating protein sorting. The formation of the actin patches has been shown to require the function of the WASH complex - the major endosomal actin polymerisation-promoting complex - which stimulates the activity of the ubiquitously expressed Arp2/3 complex. Another key component of the endosomal protein-sorting machinery is the retromer complex. Studies now show that retromer mediates the recruitment of the WASH complex and its regulators to endosomes. In this review, recent progress in understanding the role of the WASH complex along with retromer in endosomal protein sorting is discussed.
Keywords: Fam21; WASH complex; actin; endosome; retromer; sorting.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
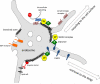
Figure 1. The role of actin patches on endosomes
Schematic diagram of a sorting/recycling endosome where retromer and the WASH complex operate. Branched actin patches formed by the action of the WASH complex demarcate discrete domains into which specific proteins are sorted for transport to their respective destinations, for example, sorting for recycling to the cell surface of proteins such as the β2-adrenergic receptor requires the WASH complex along with SNX27 and retromer. The FERM domain of SNX27 can bind to Ras potentially linking sorting to signalling [47]. Actin patches close to sites where membrane tubules are formed may contribute to scission of tubules. Additionally, myosin motors could employ the actin filaments to facilitate short-range movement and possibly tubule elongation.
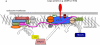
Figure 2. The key role of the Fam21 protein in linking the WASH complex to retromer
A. Cartoon diagram of the retromer and WASH complexes. The retromer cargo-selective complex (CSC) comprising VPS35, VPS29 and VPS26 function as a unit that is recruited to endosomes by RAB7a and SNX3 [14]. The TBC1D5 protein acts antagonistically to RAB7a to downregulate recruitment of the retromer CSC [32]. Through an interaction between VPS35 and FAM21 mediated by numerous LFa motifs within the long unstructured tail of FAM21, the retromer CSC recruits the WASH complex to endosomes to drive formation of branched actin patches. Additionally, MAGE-L2 connects VPS35 to TRIM27 which polyubiquitinates WASH1 and activates its NPF activity toward Arp2/3. B. Alignment of human and chicken FAM21 sequences and also human and Dictyostelium (Dicty.) FAM21 sequences showing the position of the globular head and unstructured tail domains which contain the CP-binding region and the LFa motifs (black triangles). This graphically demonstrates the evolutionary divergence of the FAM21 protein resulting in a lack of homology between human and Dictyostelium FAM21 proteins. The alignment was performed using the SIM alignment tool (
).
Figure 2. The key role of the Fam21 protein in linking the WASH complex to retromer
A. Cartoon diagram of the retromer and WASH complexes. The retromer cargo-selective complex (CSC) comprising VPS35, VPS29 and VPS26 function as a unit that is recruited to endosomes by RAB7a and SNX3 [14]. The TBC1D5 protein acts antagonistically to RAB7a to downregulate recruitment of the retromer CSC [32]. Through an interaction between VPS35 and FAM21 mediated by numerous LFa motifs within the long unstructured tail of FAM21, the retromer CSC recruits the WASH complex to endosomes to drive formation of branched actin patches. Additionally, MAGE-L2 connects VPS35 to TRIM27 which polyubiquitinates WASH1 and activates its NPF activity toward Arp2/3. B. Alignment of human and chicken FAM21 sequences and also human and Dictyostelium (Dicty.) FAM21 sequences showing the position of the globular head and unstructured tail domains which contain the CP-binding region and the LFa motifs (black triangles). This graphically demonstrates the evolutionary divergence of the FAM21 protein resulting in a lack of homology between human and Dictyostelium FAM21 proteins. The alignment was performed using the SIM alignment tool (
).
Comment in
- Response to letter by Insall.
Seaman MN, Gautreau A, Billadeau DD. Seaman MN, et al. Trends Cell Biol. 2013 Nov;23(11):520-1. doi: 10.1016/j.tcb.2013.07.012. Epub 2013 Aug 26. Trends Cell Biol. 2013. PMID: 23988428 No abstract available. - WASH complex conservation: don't WASH away the family!
Insall RH. Insall RH. Trends Cell Biol. 2013 Nov;23(11):519-20. doi: 10.1016/j.tcb.2013.07.009. Epub 2013 Aug 28. Trends Cell Biol. 2013. PMID: 23993425 No abstract available.
Similar articles
- SWIP mediates retromer-independent membrane recruitment of the WASH complex.
Dostál V, Humhalová T, Beránková P, Pácalt O, Libusová L. Dostál V, et al. Traffic. 2023 May;24(5):216-230. doi: 10.1111/tra.12884. Epub 2023 Mar 30. Traffic. 2023. PMID: 36995008 - Actin polymerization in the endosomal pathway, but not on the Coxiella-containing vacuole, is essential for pathogen growth.
Miller HE, Larson CL, Heinzen RA. Miller HE, et al. PLoS Pathog. 2018 Apr 18;14(4):e1007005. doi: 10.1371/journal.ppat.1007005. eCollection 2018 Apr. PLoS Pathog. 2018. PMID: 29668757 Free PMC article. - Endosomal recruitment of the WASH complex: active sequences and mutations impairing interaction with the retromer.
Helfer E, Harbour ME, Henriot V, Lakisic G, Sousa-Blin C, Volceanov L, Seaman MNJ, Gautreau A. Helfer E, et al. Biol Cell. 2013 May;105(5):191-207. doi: 10.1111/boc.201200038. Epub 2013 Mar 7. Biol Cell. 2013. PMID: 23331060 - The retromer complex - endosomal protein recycling and beyond.
Seaman MN. Seaman MN. J Cell Sci. 2012 Oct 15;125(Pt 20):4693-702. doi: 10.1242/jcs.103440. Epub 2012 Nov 12. J Cell Sci. 2012. PMID: 23148298 Free PMC article. Review. - Actin-dependent endosomal receptor recycling.
Simonetti B, Cullen PJ. Simonetti B, et al. Curr Opin Cell Biol. 2019 Feb;56:22-33. doi: 10.1016/j.ceb.2018.08.006. Epub 2018 Sep 15. Curr Opin Cell Biol. 2019. PMID: 30227382 Review.
Cited by
- Identification of QTL on Chromosome 18 Associated with Non-Coagulating Milk in Swedish Red Cows.
Duchemin SI, Glantz M, de Koning DJ, Paulsson M, Fikse WF. Duchemin SI, et al. Front Genet. 2016 Apr 15;7:57. doi: 10.3389/fgene.2016.00057. eCollection 2016. Front Genet. 2016. PMID: 27148354 Free PMC article. - TLR and TNF-R1 activation of the MKK3/MKK6-p38α axis in macrophages is mediated by TPL-2 kinase.
Pattison MJ, Mitchell O, Flynn HR, Chen CS, Yang HT, Ben-Addi H, Boeing S, Snijders AP, Ley SC. Pattison MJ, et al. Biochem J. 2016 Sep 15;473(18):2845-61. doi: 10.1042/BCJ20160502. Epub 2016 Jul 11. Biochem J. 2016. PMID: 27402796 Free PMC article. - Golgi Fragmentation in Neurodegenerative Diseases: Is There a Common Cause?
Martínez-Menárguez JÁ, Tomás M, Martínez-Martínez N, Martínez-Alonso E. Martínez-Menárguez JÁ, et al. Cells. 2019 Jul 19;8(7):748. doi: 10.3390/cells8070748. Cells. 2019. PMID: 31331075 Free PMC article. Review. - Cargo induces retromer-mediated membrane remodeling on membranes.
Purushothaman LK, Ungermann C. Purushothaman LK, et al. Mol Biol Cell. 2018 Nov 1;29(22):2709-2719. doi: 10.1091/mbc.E18-06-0339. Epub 2018 Sep 6. Mol Biol Cell. 2018. PMID: 30188774 Free PMC article. - WASP family proteins: Molecular mechanisms and implications in human disease.
Kramer DA, Piper HK, Chen B. Kramer DA, et al. Eur J Cell Biol. 2022 Jun-Aug;101(3):151244. doi: 10.1016/j.ejcb.2022.151244. Epub 2022 Jun 1. Eur J Cell Biol. 2022. PMID: 35667337 Free PMC article.
References
- Rotty JD, Wu C, Bear JE. New insights into the regulation and cellular functions of the ARP2/3 complex. Nat Rev Mol Cell Biol. 2013;14(1):7–12. - PubMed
- Derivery E, Sousa C, Gautier JJ, Lombard B, Loew D, Gautreau A. The Arp2/3 activator WASH controls the fission of endosomes through a large multiprotein complex. Dev. Cell. 2009;17:712–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 100140/Wellcome Trust/United Kingdom
- R01 AI065474/AI/NIAID NIH HHS/United States
- AI065474/AI/NIAID NIH HHS/United States
- G0701444/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources