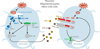The genetic theory of infectious diseases: a brief history and selected illustrations - PubMed (original) (raw)
Review
The genetic theory of infectious diseases: a brief history and selected illustrations
Jean-Laurent Casanova et al. Annu Rev Genomics Hum Genet. 2013.
Abstract
Until the mid-nineteenth century, life expectancy at birth averaged 20 years worldwide, owing mostly to childhood fevers. The germ theory of diseases then gradually overcame the belief that diseases were intrinsic. However, around the turn of the twentieth century, asymptomatic infection was discovered to be much more common than clinical disease. Paradoxically, this observation barely challenged the newly developed notion that infectious diseases were fundamentally extrinsic. Moreover, interindividual variability in the course of infection was typically explained by the emerging immunological (or somatic) theory of infectious diseases, best illustrated by the impact of vaccination. This powerful explanation is, however, best applicable to reactivation and secondary infections, particularly in adults; it can less easily account for interindividual variability in the course of primary infection during childhood. Population and clinical geneticists soon proposed a complementary hypothesis, a germline genetic theory of infectious diseases. Over the past century, this idea has gained some support, particularly among clinicians and geneticists, but has also encountered resistance, particularly among microbiologists and immunologists. We present here the genetic theory of infectious diseases and briefly discuss its history and the challenges encountered during its emergence in the context of the apparently competing but actually complementary microbiological and immunological theories. We also illustrate its recent achievements by highlighting inborn errors of immunity underlying eight life-threatening infectious diseases of children and young adults. Finally, we consider the far-reaching biological and clinical implications of the ongoing human genetic dissection of severe infectious diseases.
Figures
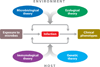
Figure 1
The four complementary theories of infectious diseases. In principle, interindividual variability of clinical presentation (ranging from asymptomatic to lethal infection) in infected individuals can depend on four sets of overlapping forces, corresponding to the microbiological, ecological, immunological, and genetic theories. Disease is attributed to microbial variation (qualitative or quantitative) in the microbiological theory; to environmental variation (other than for the pathogen concerned) in the ecological theory; to a deficiency of acquired somatic, adaptive immunity (both genetic and epigenetic elements) in the immunological theory; and to inborn errors of germline-encoded immunity (whether intrinsic, innate, or adaptive) in the genetic theory. These four theories are both complementary and overlapping.
Figure 2
Historical perspective of the pathogenesis of infectious diseases (1800–1950). The development of physiology and pathology in the early nineteenth century (Antoine Lavoisier, François Magendie, Claude Bernard), which opposed vitalism and were based on physics and chemistry, understandably led to the view that diseases were intrinsic. Compelling experimental evidence established the role of microbes (from Louis Pasteur to Robert Koch), leading to the germ theory of infectious diseases (~1870) in the most extraordinary paradigm shift ever seen in medicine. The subsequent discovery, at the turn of the twentieth century, that asymptomatic infection is much more common than disease for almost all microbes (Charles Nicolle, ~1915) only marginally modified the almost universal and deeply rooted, although only recently acquired, perception that infectious diseases are fundamentally extrinsic. Moreover, interindividual variability in the course of infection was rapidly and easily explained by the emerging immunological theory, resulting in a somatic, acquired, immunological theory of infectious diseases (from Louis Pasteur to Paul Ehrlich), best illustrated by the impact of vaccinations (Louis Pasteur, 1881). The complementary idea that human germline genetic variation could account for disease development, particularly for childhood infections lethal in the course of primary infection, was proposed early on by distinguished pioneers such as Archibald Garrod with the concept of inborn errors of immunity (~1930), the first demonstration of which was provided in the early 1950s by the descriptions of the first primary immunodeficiency (by Ogden Bruton and others) and of the protective role of the sickle cell trait against severe malaria (by Anthony Allison and others).
Figure 3
Historical perspective of the genetic theory of infectious diseases (1950–2010). We highlight eight inborn errors of immunity underlying infectious diseases that, in our view, neatly illustrate the explanatory power of a human genetic theory of childhood infectious diseases. Four are population based (blue text): protection against severe malaria (HbS trait, HBB mutation); Mendelian resistance to common Plasmodium vivax (DARC mutation) infection and to human immunodeficiency virus 1 (HIV-1) (CCR5 mutation) infection; and control of hepatitis C virus (HCV) clearance, spontaneously or with treatment, by common variants (IL28B polymorphisms). The other four are patient based (green text): primary immunodeficiencies and multiple infections [e.g., X-linked agammaglobulinemia (XLA), the first described primary immunodeficiency] and selective Mendelian susceptibilities to Epstein-Barr virus [X-linked lymphoproliferative disease (XLP)], mycobacteria [Mendelian susceptibility to mycobacterial diseases (MSMD)], and herpes simplex virus 1 (HSV-1) [herpes simplex encephalitis (HSE)] infections. The discovery of inborn errors of immunity has greatly accelerated over the past decade, owing particularly to the tremendous progress in genetic technologies.
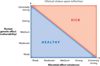
Figure 4
The respective contributions of host and microbe genetics to the clinical outcome of infectious diseases. An individual with a strong genetic vulnerability (e.g., owing to a single-gene variant) may develop clinical disease following infection with a weakly virulent microbe, whereas an individual with a low level of genetic vulnerability may develop clinical disease only if infected with a highly virulent microbe. The infection process itself is genetically controlled, with resistant and susceptible individuals.
Figure 5
A proposed age-dependent genetic architecture of infectious diseases. In this model, single-gene human variants make a major contribution to the determinism of life-threatening infectious diseases of childhood in the course of primary infection. Symptomatic reactivation and secondary infections in young adults may result from the impact of a major locus, whereas in older adults they are less influenced by human germline genetic variations (with a more complex and perhaps polygenic contribution) and probably also involve somatic factors. There is also an age-independent impact of human genetic variation on the initial step of resistance or susceptibility to the infectious process itself (e.g., DARC and Plasmodium vivax). Adapted from Reference .
Figure 6
Inborn errors of TLR3TFN-α/β-mediated immunity in childhood herpes simplex encephalitis (HSE): schematic representation of the production of and response to IFN-α/β and IFN-λ in herpes simplex virus 1 (HSV-1) immunity. HSV-1 produces viral double-stranded RNA (dsRNA) during its replication. TLR3 is a transmembrane receptor of dsRNA in the endoplasmic reticulum (ER) and endosome in most cells. In central nervous system (CNS) cells, the recognition of dsRNA by TLR3 induces activation of the IRF3 and NF-κB pathways via TRIF, leading to the production of IFN-α/β and/or IFN-λ. TLR3, UNC-93B, TRIF, TRAF3, TBK1, and NEMO deficiencies are associated with impaired IFN-α/β and/or IFN-λ production, particularly during HSV-1 infection. The binding of IFN-α/β or IFN-λ to its receptor induces the phosphorylation of JAK1 and TYK2, activating the signal transduction proteins STAT1, STAT2, and IRF9. This complex is translocated, as a heterotrimer, to the nucleus, where it acts as a transcriptional activator, binding to specific DNA response elements in the promoter region of IFN-inducible genes. STAT1 deficiencies are associated with impaired IFN-α, -β, and -λ responses. Proteins for which genetic mutations have been identified and associated with susceptibility to isolated HSE are shown in blue. Proteins for which genetic mutations have been identified and associated with susceptibility to mycobacterial, bacterial, and viral diseases (including HSE) are shown in green. Proteins for which genetic mutations have been identified but not directly associated with susceptibility to HSE are shown in red.
Similar articles
- Lethal Infectious Diseases as Inborn Errors of Immunity: Toward a Synthesis of the Germ and Genetic Theories.
Casanova JL, Abel L. Casanova JL, et al. Annu Rev Pathol. 2021 Jan 24;16:23-50. doi: 10.1146/annurev-pathol-031920-101429. Epub 2020 Apr 14. Annu Rev Pathol. 2021. PMID: 32289233 Free PMC article. Review. - Immunity and concomitant immunity in infectious diseases. Introduction.
Kallós P. Kallós P. Prog Allergy. 1982;31:IX-XV. Prog Allergy. 1982. PMID: 6755477 No abstract available. - Human genetics of infectious diseases: between proof of principle and paradigm.
Alcaïs A, Abel L, Casanova JL. Alcaïs A, et al. J Clin Invest. 2009 Sep;119(9):2506-14. doi: 10.1172/JCI38111. J Clin Invest. 2009. PMID: 19729848 Free PMC article. Review. - Immunology taught by human genetics.
Casanova JL, Abel L, Quintana-Murci L. Casanova JL, et al. Cold Spring Harb Symp Quant Biol. 2013;78:157-72. doi: 10.1101/sqb.2013.78.019968. Epub 2013 Oct 3. Cold Spring Harb Symp Quant Biol. 2013. PMID: 24092470 Review. - The human genetic determinism of life-threatening infectious diseases: genetic heterogeneity and physiological homogeneity?
Casanova JL, Abel L. Casanova JL, et al. Hum Genet. 2020 Jun;139(6-7):681-694. doi: 10.1007/s00439-020-02184-w. Hum Genet. 2020. PMID: 32462426 Free PMC article.
Cited by
- Louis Pasteur continues to shape the future of microbiology.
Mostowy S. Mostowy S. Dis Model Mech. 2022 Dec 1;15(12):dmm050011. doi: 10.1242/dmm.050011. Epub 2022 Dec 12. Dis Model Mech. 2022. PMID: 36504391 Free PMC article. - The Impact of Transmissible Microbes: How the Cystic Fibrosis Community Mobilized Against Cepacia.
Mueller R. Mueller R. Perspect Biol Med. 2023;66(1):89-106. doi: 10.1353/pbm.2023.0005. Perspect Biol Med. 2023. PMID: 38662010 Free PMC article. - Proteomics in immunity and herpes simplex encephalitis.
Pérez de Diego R, Mulvey C, Casanova JL, Godovac-Zimmermann J. Pérez de Diego R, et al. Expert Rev Proteomics. 2014 Feb;11(1):21-9. doi: 10.1586/14789450.2014.864954. Epub 2013 Dec 18. Expert Rev Proteomics. 2014. PMID: 24351021 Free PMC article. Review. - Human inborn errors of immunity: An expanding universe.
Notarangelo LD, Bacchetta R, Casanova JL, Su HC. Notarangelo LD, et al. Sci Immunol. 2020 Jul 10;5(49):eabb1662. doi: 10.1126/sciimmunol.abb1662. Sci Immunol. 2020. PMID: 32651211 Free PMC article. Review. - Human genetics of mycobacterial disease.
Dallmann-Sauer M, Correa-Macedo W, Schurr E. Dallmann-Sauer M, et al. Mamm Genome. 2018 Aug;29(7-8):523-538. doi: 10.1007/s00335-018-9765-4. Epub 2018 Aug 16. Mamm Genome. 2018. PMID: 30116885 Free PMC article. Review.
References
- Abel L, Plancoulaine S, Jouanguy E, Zhang SY, Mahfoufi N, et al. Age-dependent Mendelian predisposition to herpes simplex virus type 1 encephalitis in childhood. J. Pediatr. 2010;157:623–629. - PubMed
- Alcaïs A, Abel L, Casanova JL. Human genetics of infectious diseases. In: Speicher MR, Antonarakis SE, Motulsky AG, editors. Vogel and Motulsky’s Human Genetics: Problems and Approaches. 4th. Berlin: Springer-Verlag; 2010. pp. 403–415.
- Alcaïs A, Alter A, Antoni G, Orlova M, Van Thuc N, et al. Stepwise replication identifies a low-producing lymphotoxin-α allele as a major risk factor for early-onset leprosy. Nat. Genet. 2007;39:517–522. - PubMed
- Alcaïs A, Quintana-Murci L, Thaler DS, Schurr E, Abel L, Casanova JL. Life-threatening infectious diseases of childhood: single-gene inborn errors of immunity? Ann. N. Y. Acad. Sci. 2010;1214:18–33. - PubMed
Publication types
MeSH terms
Grants and funding
- U01 AI088685/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R37 AI095983/AI/NIAID NIH HHS/United States
- 8UL1TR000043/TR/NCATS NIH HHS/United States
- R01 AI089970/AI/NIAID NIH HHS/United States
- 5R01AI088364/AI/NIAID NIH HHS/United States
- 5R01NS072381/NS/NINDS NIH HHS/United States
- 5P01AI061093/AI/NIAID NIH HHS/United States
- UL1 TR000043/TR/NCATS NIH HHS/United States
- 5R01AI089970/AI/NIAID NIH HHS/United States
- R01 NS072381/NS/NINDS NIH HHS/United States
- 268777/ERC_/European Research Council/International
- 5U01AI088685/AI/NIAID NIH HHS/United States
- 5R37AI095983/AI/NIAID NIH HHS/United States
- R01 AI088364/AI/NIAID NIH HHS/United States
- P01 AI061093/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous