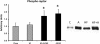The effects of chronic AMPK activation on hepatic triglyceride accumulation and glycerol 3-phosphate acyltransferase activity with high fat feeding - PubMed (original) (raw)
The effects of chronic AMPK activation on hepatic triglyceride accumulation and glycerol 3-phosphate acyltransferase activity with high fat feeding
Bradley S Henriksen et al. Diabetol Metab Syndr. 2013.
Abstract
Background: High fat feeding increases hepatic fat accumulation and is associated with hepatic insulin resistance. AMP Activated Protein Kinase (AMPK) is thought to inhibit lipid synthesis by the acute inhibition of glycerol-3-phosphate acyltransferase (GPAT) activity and transcriptional regulation via sterol regulatory element binding protein-1c (SREBP-1c).
Methods: The purpose of this study was to determine if chronic activation of AMPK prevented an increase in GPAT1 activity in rats fed a high fat diet. Rats were fed a control (C), or a high fat (HF) diet (60% fat) for 6 weeks and injected with saline or a daily aminoimidazole carboxamide ribnucleotide (AICAR) dose of 0.5 mg/g body weight.
Results: Chronic AMPK activation by AICAR injections resulted in a significant reduction in hepatic triglyceride accumulation in both the C and HF fed animals (C, 5.5±0.7; C+AICAR, 2.7 ±0.3; HF, 21.8±3.3; and HF+AICAR, 8.0±1.8 mg/g liver). HF feeding caused an increase in total GPAT and GPAT1 activity, which was not affected by chronic AMPK activation (GPAT1 activity vs. C, C+AICAR, 92±19%; HF, 186±43%; HF+AICAR, 234±62%). Markers of oxidative capacity, including citrate synthase activity and cytochrome c abundance, were not affected by chronic AICAR treatment. Interestingly, HF feeding caused a significant increase in long chain acyl-CoA dehydrogenase or LCAD (up 66% from C), a marker of fatty acid oxidation capacity.
Conclusions: These results suggest that chronic AMPK activation limits hepatic triglyceride accumulation independent of a reduction in total GPAT1 activity.
Keywords: AMPK; GPAT1; LCAD; SREBP-1c; mTOR.
Figures
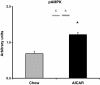
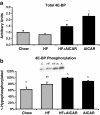
Figure 1
Phospho-AMP-activated protein kinase (pAMPK) content in the liver was increased with acute 5-aminoimidazole-4-carboxamide riboside (AICAR) treatment (n=5-7). Livers from AICAR-treated rats were removed 1 hr after injection. Asterisk (*) denotes a main effect of AICAR (p<0.05). Graph represents means ± SE.
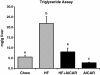
Figure 2
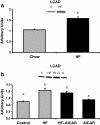
Chronic activation of liver AMPK with daily injections of AICAR limited the normal increase in triglyceride accumulation that occurs with high fat feeding such that it was not significantly different from the control group (n = 7-12). Letters are used to represent significance; same letter means no significant difference (P < 0.05). Graph represents means ± SE.
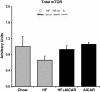
Figure 3
Total mammalian target of rapamycin complex (mTOR) protein content of liver extracts revealed no significant differences with high fat feeding or chronic AMPK activation (n = 4-5). Bands for all 4 groups were taken side by side with no interruption. Graph represents means ± SE.
Figure 4
Phospho-raptor content was increased in livers treated with AICAR (n= 4-5). Asterisk (*) denotes a main effect of AICAR (p<0.05). Graph represents means ± SE.
Figure 5
Eukaryotic initiation factor 4E-binding protein (4EBP) following HF and AICAR treatments. a. Total Eukaryotic initiation factor 4E-binding protein (4EBP) results show an increase with the treatment of AICAR (n = 4-5). Bands for all 4 groups were taken side by side with no interruption Asterisk (*) denotes a main effect of AICAR treatment (P<0.05). Graph represents means ± SE. b Eukaryotic initiation factor 4E-binding protein (4EBP) phosphorylation (percentage of the total protein in the 2 hypophosphorylated bands compared to total) showed an increased phosphorylation with AICAR treatment (n = 4-5). Bands for all 4 groups were taken side by side with no interruption. Letters are used to represent significance; same letter means no significant difference (P < 0.05). Graph represents means ± SE.
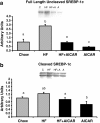
Figure 6
Chronic activation of AMPK and sterol regulatory element binding protein-1c (SREBP-1c). a. Chronic activation of AMPK caused a reduction in the total abundance of uncleaved Sterol regulatory element binding protein-1c (SREBP-1c) in rats fed either chow or high fat diet (n = 3-5). Bands for all 4 groups were taken side by side with no interruption. Letters are used to represent significance. A significant main effect of AICAR was observed (p<0.05). Graph represents means ± SE. b. Chronic activation of AMPK caused a reduction in the total abundance of cleaved (65-68 kDa bands) SREBP-1c in the liver of rats fed either chow or high fat diet (n = 4-5). Bands for all 4 groups were taken side by side with no interruption. Letters are used to represent significance. A significant main effect of AICAR and high fat feeding was observed (p<0.05). Graph represents means ± SE.
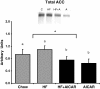
Figure 7
Total acetyl coA carboxylase (ACC) content had a main effect of chronic AMPK activation (n = 7-10). High fat feeding blunted the decrease in total ACC content with the HF + AICAR group. Bands for all 4 groups were taken side by side with no interruption. Letters are used to represent significance. Asterisk (*) denotes a main effect of AICAR (P < 0.05). Graph represents means ± SE.
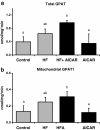
Figure 8
GPAT activity following high fat feeding and AICAR treatments. a. High fat feeding increased total glycerol-3-phosphate acyl-transferase (GPAT) activity (n = 5-8) in liver. A main AICAR effect on total GPAT activity was absent. Asterisk (*) denotes a main effect of high fat feeding (P < 0.05). Graph represents means ± SE. b. High fat feeding increased NEM-sensitive glycerol-3-phosphate acyl-transferase (GPAT1) activity in liver (n = 5-8). A main AICAR effect on total GPAT activity was absent. *Main treatment effect0020 (P < 0.05). Graph represents means ± SE.
Figure 9
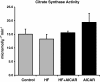
Citrate synthase activity in the liver did not increase with either high fat feeding or chronic AMPK activation (n = 4-5). Graph represents means ± SE.
Figure 10
Long chain acyl CoA dehydrogenase (LCAD) following high fat feeding and AICAR treatments. a. There was a main high fat effect on total long chain acyl CoA dehydrogenase (LCAD) content in the liver (n = 8-10). Asterisk (*) indicates a main effect of high fat diet (P < 0.05). Graph represents means ± SE. b. Chronic activation of AMPK did not have an effect on total long chain acyl CoA dehydrogenase (LCAD) content in the liver (n = 4-5). Bands for all 4 groups were taken side by side with no interruption. Letters are used to represent significance; same letter means no significant difference (P < 0.05). Graph represents means ± SE.
Similar articles
- Transcriptional Regulation of Acyl-CoA:Glycerol-_sn_-3-Phosphate Acyltransferases.
Karasawa K, Tanigawa K, Harada A, Yamashita A. Karasawa K, et al. Int J Mol Sci. 2019 Feb 22;20(4):964. doi: 10.3390/ijms20040964. Int J Mol Sci. 2019. PMID: 30813330 Free PMC article. Review. - Increased dietary fat contributes to dysregulation of the LKB1/AMPK pathway and increased damage in a mouse model of early-stage ethanol-mediated steatosis.
Shearn CT, Smathers RL, Jiang H, Orlicky DJ, Maclean KN, Petersen DR. Shearn CT, et al. J Nutr Biochem. 2013 Aug;24(8):1436-45. doi: 10.1016/j.jnutbio.2012.12.002. Epub 2013 Mar 1. J Nutr Biochem. 2013. PMID: 23465594 Free PMC article. - Chronic AMP-activated protein kinase activation and a high-fat diet have an additive effect on mitochondria in rat skeletal muscle.
Fillmore N, Jacobs DL, Mills DB, Winder WW, Hancock CR. Fillmore N, et al. J Appl Physiol (1985). 2010 Aug;109(2):511-20. doi: 10.1152/japplphysiol.00126.2010. Epub 2010 Jun 3. J Appl Physiol (1985). 2010. PMID: 20522731 Free PMC article. - AICAR, an AMPK activator, has protective effects on alcohol-induced fatty liver in rats.
Tomita K, Tamiya G, Ando S, Kitamura N, Koizumi H, Kato S, Horie Y, Kaneko T, Azuma T, Nagata H, Ishii H, Hibi T. Tomita K, et al. Alcohol Clin Exp Res. 2005 Dec;29(12 Suppl):240S-5S. doi: 10.1097/01.alc.0000191126.11479.69. Alcohol Clin Exp Res. 2005. PMID: 16385230 Clinical Trial. - Regulation of Triglyceride Metabolism. II. Function of mitochondrial GPAT1 in the regulation of triacylglycerol biosynthesis and insulin action.
Gonzalez-Baró MR, Lewin TM, Coleman RA. Gonzalez-Baró MR, et al. Am J Physiol Gastrointest Liver Physiol. 2007 May;292(5):G1195-9. doi: 10.1152/ajpgi.00553.2006. Epub 2006 Dec 7. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17158253 Free PMC article. Review.
Cited by
- Transcriptional Regulation of Acyl-CoA:Glycerol-_sn_-3-Phosphate Acyltransferases.
Karasawa K, Tanigawa K, Harada A, Yamashita A. Karasawa K, et al. Int J Mol Sci. 2019 Feb 22;20(4):964. doi: 10.3390/ijms20040964. Int J Mol Sci. 2019. PMID: 30813330 Free PMC article. Review. - Inhibitory Effects of Twenty-Nine Compounds From Potentilla longifolia on Lipid Accumulation and Their Mechanisms in 3T3-L1 Cells.
Ma Q, Ye L, Li W, Lin S, Zhao X, Jin C, Liu G, Liu H, Sun Y, Yuan H, Piao G. Ma Q, et al. Front Pharmacol. 2020 Nov 9;11:555715. doi: 10.3389/fphar.2020.555715. eCollection 2020. Front Pharmacol. 2020. PMID: 33240084 Free PMC article. - Hepatic AKAP1 deficiency exacerbates diet-induced MASLD by enhancing GPAT1-mediated lysophosphatidic acid synthesis.
He L, She X, Guo L, Gao M, Wang S, Lu Z, Guo H, Li R, Nie Y, Xing J, Ji L. He L, et al. Nat Commun. 2025 May 8;16(1):4286. doi: 10.1038/s41467-025-58790-7. Nat Commun. 2025. PMID: 40341440 Free PMC article. - Effect of a high fat, high sucrose diet on the promotion of non-alcoholic fatty liver disease in male rats: the ameliorative role of three natural compounds.
Ragab SM, Abd Elghaffar SKh, El-Metwally TH, Badr G, Mahmoud MH, Omar HM. Ragab SM, et al. Lipids Health Dis. 2015 Jul 31;14:83. doi: 10.1186/s12944-015-0087-1. Lipids Health Dis. 2015. PMID: 26228038 Free PMC article. - AICAR and Metformin Exert AMPK-dependent Effects on INS-1E Pancreatic β-cell Apoptosis via Differential Downstream Mechanisms.
Dai YL, Huang SL, Leng Y. Dai YL, et al. Int J Biol Sci. 2015 Sep 14;11(11):1272-80. doi: 10.7150/ijbs.12108. eCollection 2015. Int J Biol Sci. 2015. PMID: 26435693 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous