The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression - PubMed (original) (raw)
The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression
Laura M Koontz et al. Dev Cell. 2013.
Abstract
The Hippo tumor suppressor pathway restricts tissue growth by inactivating the transcriptional coactivator Yki. Although Sd has been implicated as a DNA-binding transcription factor partner for Yki and can genetically account for gain-of-function Yki phenotypes, how Yki regulates normal tissue growth remains a long-standing puzzle because Sd, unlike Yki, is dispensable for normal growth in most Drosophila tissues. Here we show that the yki mutant phenotypes in multiple developmental contexts are rescued by inactivation of Sd, suggesting that Sd functions as a default repressor and that Yki promotes normal tissue growth by relieving Sd-mediated default repression. We further identify Tgi as a cofactor involved in Sd's default repressor function and demonstrate that the mammalian ortholog of Tgi potently suppresses the YAP oncoprotein in transgenic mice. These findings fill a major gap in Hippo-mediated transcriptional regulation and open up possibilities for modulating the YAP oncoprotein in cancer and regenerative medicine.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
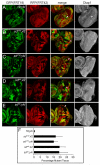
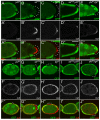
Figure 1. Yki promotes normal growth by relieving Sd-mediated default repression in the eye
(A–E) Eye discs containing mutant clones of the indicated genotypes were stained with α-Diap1. Note the small size and decreased Diap1 expression of 19; yki clones (A), but not sd; 42 (B and D) or sd; yki clones (C and E) (compare arrowheads). (F) Quantification of the relative percentage of the mutant tissues in the eye (mean ± SEM). At least 12 eye discs were blindly scored for each genotype. Note the comparable areas occupied by sd; 42 and sd; yki mutant tissues.
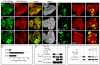
Figure 2. Tgi specifically represses Hippo target genes and functions epistatically to Wts
(A–D) Eye discs containing GFP-marked Tgi Flp-out clones (arrowheads), showing decreased diap1-lacZ (A) or ex-lacZ (B), and normal expression of Eya (C) or Arm (D). (E–H) Wing discs containing GFP-marked MARCM clones of control (E), Tgi overexpression (F), wts mutant (G), and wts mutant with Tgi overexpression (H), and stained for Diap1 protein. See Figure S1, S2 and Table S1 for data supplemental to Figure 2.
Figure 3. Tgi's growth suppressive function requires Sd
(A) Alignment of the Tondu domains from Drosophila Vg and Tgi and human Vgl4 (left), and co-IP between Tgi and Sd (right). Tondu domain mutation was made by changing the conserved D/ED/EHF sequence at the core of the domain to AAAA. For co-IP, the indicated epitope-tagged constructs were expressed in S2R+ cells and subjected to IP as indicated. (B) Co-IP between Yki and wildtype Tgi or Tgi mutants with single, double or triple PPxY mutations. PPxY motif was mutated by changing Tyr to Ala. Note that mutation of P1, but not P2 or P3, abolished Tgi-Yki interactions, and all mutants containing P1 (P1, P12, P13 and P123) showed a similar abolishment of Tgi-Yki interactions. Also note that mutation of both Tondu domains (T12) did not affect Tgi-Yki binding. (C) Wildtype or Tgi mutants were expressed by _nub_-Gal4. Representative adult wings (if wings were present) or whole flies (if no wings were present) are shown. The graph shows quantification of wing size relative to nub-Gal4/+ control (mean ± SEM). ND: not determined due to a no-wing phenotype. (D–F) Eye discs containing GFP-marked MARCM clones of Tgi overexpression (D), sd mutant (E) or sd mutant with Tgi overexpression (F), and stained for diap1-lacZ. (G) Tgi binds to the HRE of diap1 in an Sd-dependent manner. At the top is a schematic representation of the diap1 locus, showing exon 1 (Ex1) and exon 2 (Ex2) of the RA transcript, as well as the previously defined HRE (Wu et al., 2008). Chromatins were precipitated from lysates of S2R+ cells expressing the indicated combination of HA-Sd and FLAG-Tgi plasmids using anti-FLAG antibody. A ~100bp DNA (+3993–+4101) encompassing the HRE was amplified following ChIP. The region −3536 to −3430 (marked as 5'con) was used as a negative control region for PCR. The HRE amplicon was enriched only in the presence of both FLAG-Tgi and HA-Sd. See Figure S3 for data supplemental to Figure 3.
Figure 4. Tgi is required for default repression but not for Yki-mediated activation
(A–D) Loss of tgi partially rescues yki loss-of-function phenotypes. Eye discs containing mutant clones of the indicated genotypes were stained with α-Diap1 (A–C). The areas occupied by the mutant tissues were quantified in (D) (mean ± SEM). 42D _yki_B5; 80B _tgi_ΔP mutant clones were significantly larger than 42D _yki_B5; 80B+ mutant clones, but still statistically smaller than 42D+; 80B _tgi_ΔP mutant clones (p= 0.00016), and still showed decreased Diap1 expression (arrowheads). (E–G) Tgi is not required for Yki-mediated activation. Eye discs (E–F) and wing disc (G) containing MARCM clones (GFP-positive) of the indicated genotypes were stained for Diap1. Yki-overexpressing clones with or without tgi mutation showed similar round clone shape and elevated Diap1 levels. (H) Yki inhibits Sd-Tgi binding in cultured cells. The indicated plasmids were transfected into S2R+ cells and subjected to immunoprecipitation by anti-FLAG. HA-Yki, but not HA-YkiP88L (Wu et al., 2008) inhibited Sd-Tgi interactions. (I) Yki inhibits Sd-Tgi binding in vitro. Lanes 1–2: FLAG-Tgi expressed in S2R+ cells was immunoprecipitated with anti-FLAG conjugated beads. Beads with or without FLAG-Tgi were incubated with bacterially purified CFP-Sd protein, and the amount of CFP-Sd bound by the beads were probed with α-CFP antibody. CFP-Sd was recovered only by beads with FLAG-Tgi. Lanes 3–5: similar to lane 2, except that increasing concentration of bacterially purified Yki (0x, 0.5x and 1x relative to CFP-Sd, lanes 3–5) was added to the incubation mixture. Increasing concentration of Yki decreased the amount of CFP-Sd bound to FLAG-Tgi conjugated beads. See Figure S4 for data supplemental to Figure 4.
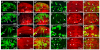
Figure 5. Default repression mediated by Sd and Tgi in ovarian follicle cells
(A–E) Loss of sd or tgi results in persistent Cut expression in stage 7–9 PFCs. Mutant clones of the indicated genotypes were marked as GFP-negative and stained for Cut. PFC clones are highlighted by white dots. (A–C) stage 7 egg chambers. Note the persistent expression of Cut in PFC clones of sd or tgi, but not yki. (D–E) stage 9 egg chambers, showing persistent Cut expression in sd; yki or yki; tgi double mutant PFC clones. (F–J) Loss of sd or tgi rescues yki mutant phenotype in early stage follicle cells. Mosaic stage 5 egg chambers were stained for Cut. Mutant clones of the respective genotypes were marked as GFP-negative and highlighted by white dots. Note the reduced Cut expression in yki clones, but not sd, tgi, sd; yki, or yki; tgi clones. See Figure S5 for data supplemental to Figure 5.
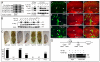
Figure 6. Sd and Tgi are required for ectopic apoptosis induced by hyperactive Hippo signaling
(A–G) Eye discs were stained for TUNEL (red) and DAPI (blue) (A–F), and TUNEL positive nuclei were quantified (G) (mean ± SEM, n=8). TUNEL positive nuclei in the whole eye disc were counted by projecting a Z-stack of serial confocal optical sections into one focal plane. *** denotes a p-value < 1.5E-5. The complete genotypes are: (A) UAS-Dicer2; GMR-Gal4; (B) UAS-Dicer2; GMR-Gal4 UAS-Ex; UAS-GFPRNAi; (C) UAS-Dicer2; GMR-Gal4; UAS-Sd RNAi; (D) UAS-Dicer2; GMR-Gal4 UAS-Ex; UAS-SdRNAi; (E) UAS-Dicer2; GMR-Gal4/UAS- TgiRNAi; (F) UAS-Dicer2; GMR-Gal4 UAS-Ex/UAS-TgiRNAi. (H–L) Wing discs were stained for TUNEL and DAPI (H–K) and TUNEL positive nuclei in the wing pouch region was quantified by Z-stack projections of confocal sections (L). The complete genotypes are: (H) MS1096-Gal4 UAS-Flp; UAS-Hpo; (I) MS1096-Gal4 UAS-Flp; UAS-Hpo; FRT80B_tgi_ΔP/FRT80 RpS17; (J) UAS-Dicer2; nub-Gal4/UAS-Hpo; (K) UAS-Dicer2; nub-Gal4/UAS-Hpo; UAS-TgiRNAi. (L) shows quantification of TUNEL-positive nuclei in the wing pouch (mean ± SEM, n=8). *** denotes a p-value < 2.5E-5. (M) and (N): Adult flies corresponding to animals analyzed in (H–I) and (J–K), respectively. Note the extremely reduced wing size in flies expressing UAS-Hpo by MS1096-Gal4 or nub-Gal4 (animal to the left; showing tiny wing remnants), and the appreciable wing size of flies in which Hpo overexpression was combined with loss of Tgi (animal to the right). (O) A model for how Hippo signaling switches Sd between Tgi-mediated repression and Yki-mediated activation. Additional Sd co-repressors (protein X in the diagram) likely exist since Tgi plays a partial role in Sd-mediated default repression in the eye disc. See text for details. See Figure S6 for data supplemental to Figure 6.
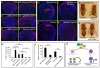
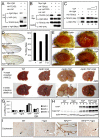
Figure 7. Conserved function of Vgl-4 in Hippo signaling and organ size control
(A) Vgl4 binds to TEAD2 but not YAP. The indicated plasmids were transfected into HEK293 cells and analyzed by immunoprecipitation. (B) YAP inhibits TEAD2-Vgl4 binding in cultured cells. The indicated plasmids were transfected into HEK293 cells and subjected to immunoprecipitation by anti-Myc. HA-YAP, but not the TEAD binding-defective HA-YAPS94A (Zhao et al., 2008), inhibited TEAD2-Vgl4 interactions. (C) YAP inhibits in vitro binding between TEAD2 and Vgl4. Lanes 1–2: HA-Vgl4 expressed in HEK293 cells was immunoprecipitated on Protein G beads. Protein G beads with or without HA-Vgl4 were incubated with purified His-TEAD2, and the amount of His-TEAD2 bound by the beads were probed with α-His antibody. His-TEAD2 was recovered only by protein G beads with HA-Vgl4 (compare lanes 1 and 2). Lanes 3–5: similar to lane 2, except that increasing concentration of purified YAP (0×, 0.5× and 1× relative to TEAD2, lanes 3–5) was added to the incubation mixture. Increasing concentration of YAP decreased the amount of TEAD2 bound to HA-Vgl4 conjugated protein G beads. (D) _nub_-Gal4 was used to express _attB_-UAS-Vgl4 or _attB_-UAS-Vgl4T12 inserted into the same chromosomal locus. The graph shows quantification of wing size relative to _nub_-Gal4/+ control (mean ± SEM, n=8). Vgl4-expressing wings were smaller than control (69 ± 1% vs.100 ± 1%; p=3.4E-7). Vgl4T12-expressing wings were slighted smaller than control (95 ± 1% vs.100 ± 1%; p-value: 0.004). (E) Vgl4 suppressed eye overgrowth induced by YAP127A in Drosophila. (F) livers from wildtype (WT), ApoE-rtTA/TRE-Vgl4 (ApoE>Vgl4), ApoE-rtTA/TRE-YAP (ApoE>YAP), ApoE-rtTA/TRE-YAP/TRE-Vgl4 (ApoE>YAP+Vgl4) mice, treated with 0.2g/L Dox for 4 weeks starting at 3 weeks of age (4W0.2, top row), or 1g/L Dox for 8 weeks starting at birth (8WB1.0, bottom row). Note the enlarged size of ApoE>YAP livers in both conditions and the widespread development of HCC (arrowheads) in 8WB1.0 condition. Both were suppressed in ApoE>YAP+Vgl4 livers. (G) Quantification of liver-to-body weight ratio for animals analyzed in (F). Besides 4W0.2 and 8WB1.0, an additional condition in which animals were treated with 0.2g/L Dox for 2 weeks starting at 3 weeks of age (abbreviated as 2W0.2) is also included. Values are mean ± SEM, n>=3 for each data point. (H) Following 2 weeks of 0.2 g/L Dox treatment starting at 3 weeks of age, liver lysates from the indicated genotypes were probed with an antibody specific to human YAP (top gel), an antibody reactive to both mouse and human YAP (middle gel) and an antibody against actin (bottom gel). Three mice were analyzed for each genotype. (I) Vgl4 suppressed _Nf2_-deficient phenotype in the liver. Littermates of the following genotypes were treated with Dox starting from E11.5 and analyzed at birth with CK staining (arrowheads). WT: ROSA26-loxP-STOP-loxP-rtTA/+; NF2flox2/+ >Vgl4: Alb-Cre; Nf2flox2/flox2; ROSA26-loxP-STOP-loxP-rtTA; TRE-Vgl4 NF2CKO: Alb-Cre; Nf2flox2/flox2; ROSA26-loxP-STOP-loxP-rtTA/ ROSA26-loxP-STOP-loxP-rtTA NF2CKO >Vgl4: Alb-Cre; Nf2flox2/flox2; ROSA26-loxP-STOP-loxP-rtTA; TRE-Vgl4 See Figure S7 and Table S2 for data supplemental to Figure 7.
Similar articles
- Brahma regulates the Hippo pathway activity through forming complex with Yki-Sd and regulating the transcription of Crumbs.
Zhu Y, Li D, Wang Y, Pei C, Liu S, Zhang L, Yuan Z, Zhang P. Zhu Y, et al. Cell Signal. 2015 Mar;27(3):606-13. doi: 10.1016/j.cellsig.2014.12.002. Epub 2014 Dec 8. Cell Signal. 2015. PMID: 25496831 - SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila.
Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. Goulev Y, et al. Curr Biol. 2008 Mar 25;18(6):435-41. doi: 10.1016/j.cub.2008.02.034. Epub 2008 Feb 28. Curr Biol. 2008. PMID: 18313299 - A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity.
Guo T, Lu Y, Li P, Yin MX, Lv D, Zhang W, Wang H, Zhou Z, Ji H, Zhao Y, Zhang L. Guo T, et al. Cell Res. 2013 Oct;23(10):1201-14. doi: 10.1038/cr.2013.120. Epub 2013 Sep 3. Cell Res. 2013. PMID: 23999857 Free PMC article. - A role for Hipk in the Hippo pathway.
Heidary Arash E, Attisano L. Heidary Arash E, et al. Sci Signal. 2013 May 14;6(275):pe18. doi: 10.1126/scisignal.2004259. Sci Signal. 2013. PMID: 23674821 Review. - Crosstalk between the mTOR and Hippo pathways.
Honda D, Okumura M, Chihara T. Honda D, et al. Dev Growth Differ. 2023 Aug;65(6):337-347. doi: 10.1111/dgd.12867. Epub 2023 Jun 14. Dev Growth Differ. 2023. PMID: 37209252 Review.
Cited by
- Interaction of the Hippo Pathway and Phosphatases in Tumorigenesis.
Sarmasti Emami S, Zhang D, Yang X. Sarmasti Emami S, et al. Cancers (Basel). 2020 Aug 27;12(9):2438. doi: 10.3390/cancers12092438. Cancers (Basel). 2020. PMID: 32867200 Free PMC article. Review. - Scalloped and Yorkie are required for cell cycle re-entry of quiescent cells after tissue damage.
Meserve JH, Duronio RJ. Meserve JH, et al. Development. 2015 Aug 15;142(16):2740-51. doi: 10.1242/dev.119339. Epub 2015 Jul 9. Development. 2015. PMID: 26160905 Free PMC article. - TNS1: Emerging Insights into Its Domain Function, Biological Roles, and Tumors.
Wang Z, Ye J, Dong F, Cao L, Wang M, Sun G. Wang Z, et al. Biology (Basel). 2022 Oct 26;11(11):1571. doi: 10.3390/biology11111571. Biology (Basel). 2022. PMID: 36358270 Free PMC article. Review. - Disease implication of hyper-Hippo signalling.
Wang SP, Wang LH. Wang SP, et al. Open Biol. 2016 Oct;6(10):160119. doi: 10.1098/rsob.160119. Open Biol. 2016. PMID: 27805903 Free PMC article. Review. - The Effect of Long-Term Passage on Porcine SMCs' Function and the Improvement of TGF-β1 on Porcine SMCs' Secretory Function in Late Passage.
Zheng YY, Hu ZN, Liu Z, Jiang YC, Guo RP, Ding SJ, Zhou GH. Zheng YY, et al. Foods. 2023 Jul 12;12(14):2682. doi: 10.3390/foods12142682. Foods. 2023. PMID: 37509774 Free PMC article.
References
- Affolter M, Pyrowolakis G, Weiss A, Basler K. Signal-induced repression: the exception or the rule in developmental signaling? Dev. Cell. 2008;15:11–22. - PubMed
- Barolo S, Posakony JW. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16:1167–1181. - PubMed
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous