The Bardet-Biedl syndrome-related protein CCDC28B modulates mTORC2 function and interacts with SIN1 to control cilia length independently of the mTOR complex - PubMed (original) (raw)
The Bardet-Biedl syndrome-related protein CCDC28B modulates mTORC2 function and interacts with SIN1 to control cilia length independently of the mTOR complex
Magdalena Cardenas-Rodriguez et al. Hum Mol Genet. 2013.
Abstract
CCDC28B encodes a coiled coil domain-containing protein involved in ciliogenesis that was originally identified as a second site modifier of the ciliopathy Bardet-Biedl syndrome. We have previously shown that the depletion of CCDC28B leads to shortened cilia; however, the mechanism underlying how this protein controls ciliary length is unknown. Here, we show that CCDC28B interacts with SIN1, a component of the mTOR complex 2 (mTORC2), and that this interaction is important both in the context of mTOR signaling and in a hitherto unknown, mTORC-independent role of SIN1 in cilia biology. We show that CCDC28B is a positive regulator of mTORC2, participating in its assembly/stability and modulating its activity, while not affecting mTORC1 function. Further, we show that Ccdc28b regulates cilia length in vivo, at least in part, through its interaction with Sin1. Importantly, depletion of Rictor, another core component of mTORC2, does not result in shortened cilia. Taken together, our findings implicate CCDC28B in the regulation of mTORC2, and uncover a novel function of SIN1 regulating cilia length that is likely independent of mTOR signaling.
Figures
Figure 1.
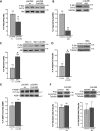
CCDC28B interacts with the mTORC2 components SIN1 and Rictor. (A) SIN1 confers yeast cells the ability to grow at the restrictive temperature of 37°C only in the presence of CCDC28B. MAFB–MAFB: positive control; MAFB–Lamin C, ColI-MAFB and pSOS ev–pMyr ev: negative controls. (B) Myc-SIN1.1 (long isoform) is detected after immunoprecipitating HA-CCDC28B (left). Myc-CCDC28B is also detected after immunoprecipitating with α-SIN1 antibody (right). Same cell lysates were used for IP controls and α-SIN1 thus controlling for initial levels of SIN1. (C) Rictor, but not Raptor or mTOR, is co-immunoprecipitated with Myc-CCDC28B. Same conditions were used in all cases. Molecular weights are indicated in kDa.
Figure 2.
CCDC28B modulates mTORC2 activity. (A and B) Depletion of CCDC28B in both NIH3T3 cells (A) and zebrafish embryos (B) results in a reduction of phospho-Akt S473 indicating decreased mTORC2 activity. Total Akt or GAPDH were used as loading controls. (C and D) Overexpression of CCDC28B in cells (C) and in zebrafish (D) resulted in increased mTORC2 activity. Total Akt and tubulin were used as controls. (E and F) Depletion of Ccdc28b in cells (E) or in vivo (F) did not affect the activity of mTORC1, measuring P-4EBP1 (T37/46) or P-S6 (S235/236 and S240/244), respectively. Total 4EBP1 and Tubulin were used as loading controls. Asterisks indicate statistically significant differences (P < 0.01). Error bars correspond to SEM. Molecular weights are indicated in kDa.
Figure 3.
CCDC28B plays a role in mTORC2 assembly and/or stability. Immunoprecipitations using α-SIN1 from both control and CCDC28B knockdown cells (stealth) showed a significant reduction in the amounts of mTOR (A) and Rictor (B) in complex with SIN1. In contrast, immunoprecipitations with α-Raptor demonstrated that the interaction between Raptor–mTOR was not affected (C). The quantification of the western blot bands is shown (D). Asterisks mark significant differences (P < 0.01). Error bars correspond to SD. Molecular weights are indicated in kDa.
Figure 4.
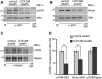
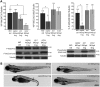
Knockdown of mTORC1 and mTORC2 components in zebrafish. (A) The activity of mTORC1 (P-S6 S235/236 and S240/244) and mTORC2 (P-Akt S473) was measured in each morphant. Knockdown of Raptor affected mTORC1 activity, while knockdown of Sin1 and Rictor resulted in reduced P-Akt S473. Tubulin was used as loading control. The results correspond to two independent injections. In each experiment, 25–30 embryos per condition were pooled to extract protein. 4 ng of MOstd were used in all cases. Asterisks denote statistically significant differences (P < 0.01). Error bars correspond to SEM. Representative westerns are shown and molecular weights are indicated in kDa. (B) Representative images of embryos injected with the control MOstd, sin1, raptor and rictor MOspl. Raptor morphants are characterized by a curved and shortened body axis, craniofacial defects and alterations in pigmentation (arrow). Sin1 morphants are shorter than controls and present reduced cranial length and width. Rictor morphants have no obvious morphological phenotypes despite a marked reduction in mTORC2 activity. Embryos were assessed at 48 hpf. All images were taken using the same magnification.
Figure 5.
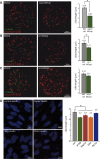
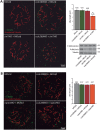
Sin1 affects cilia length independently of mTORC2. 18–20 KVs of each raptor (A) sin1 (B) and rictor (C) splice morphants were analyzed by immunofluorescence and confocal microscopy using a γ-tubulin (green) and acetylated α-tubulin (red) antibodies to stain basal bodies and cilia, respectively. A standard MO (MOstd) was injected as control alongside each morpholino injection. In excess of 500 cilia were analyzed for each condition and the length of cilia was measured using ImageJ. The average cilia length is shown in the graphs. Both, knockdown of Raptor (A) and Sin1 (B), but not Rictor (C), resulted in a significant (asterisks) shortening of KV cilia. Error bars correspond to SEM; P < 0.001. (D) Cilia length was measured in hTERT-RPE cells transfected with stealth double-stranded RNAi oligos. Cilia and basal bodies were labeled using acetylated α-tubulin (green) and γ-tubulin (red), respectively. In excess of 400 cilia were analyzed per condition using ImageJ. Again, knockdown of Raptor and SIN1, but not Rictor, resulted in significantly (asterisk) shortened cilia. Knockdown of IFT88 was used as a control of the system. Error bars correspond to SEM; P < 0.001.
Figure 6.
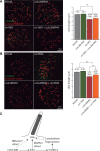
ccdc28b and sin1 interact genetically. Sub-optimal doses of ccdc28b, sin1 and raptor MOspl were used to test for genetic interactions between these genes. (A) Injection of either the control morpholino (MOstd), 2 ng of ccdc28b MOspl or 2 ng of sin1 MOspl did not result in cilia shortening. However, co-injecting these suboptimal doses of ccdc28b and sin1 MOspl resulted in significantly shorter KV cilia. The asterisk denotes statistically significant differences (P < 0.001). Importantly, this effect on cilia length is not explained by mTORC1 (P-S6 S240/244) or mTORC2 (P-Akt S473) dysfunction (western blots). Molecular weights are expressed in kDa. (B) In contrast, neither single nor double injections of the ccdc28b and raptor MOspl affected cilia length. Error bars correspond to SEM.
Figure 7.
Ccdc28b affects ciliary length upstream of SIN1. Reciprocal rescue experiments to further characterize the ccdc28b-sin1 interaction. (A) The KV cilia phenotype of sin1 full dose morphants was not rescued by co-injection of ccdc28b mRNA. Also, overexpression of ccdc28b by mRNA injection did not affect cilia length by itself. (B) In contrast, while overexpression of sin1 alone did not affect ciliary length, injecting sin1 mRNA in ccdc28b morphants partially restored the length of KV cilia. The quantifications of average cilia length for each condition are shown. Asterisks denote statistically significant differences (P < 0.001). Error bars correspond to SEM. (C) Model summarizing the results. CCDC28B regulates cilia length at least in part through SIN1. In addition, CCDC28B modulates the activity of SIN1 in the context of mTORC2, acting as an agonist of the complex. Dashed lines indicate alternatives mentioned in the discussion.
Similar articles
- Kinesin 1 regulates cilia length through an interaction with the Bardet-Biedl syndrome related protein CCDC28B.
Novas R, Cardenas-Rodriguez M, Lepanto P, Fabregat M, Rodao M, Fariello MI, Ramos M, Davison C, Casanova G, Alfaya L, Lecumberry F, González-Sapienza G, Irigoín F, Badano JL. Novas R, et al. Sci Rep. 2018 Feb 14;8(1):3019. doi: 10.1038/s41598-018-21329-6. Sci Rep. 2018. PMID: 29445114 Free PMC article. - PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor beta expression and signaling.
Woo SY, Kim DH, Jun CB, Kim YM, Haar EV, Lee SI, Hegg JW, Bandhakavi S, Griffin TJ, Kim DH. Woo SY, et al. J Biol Chem. 2007 Aug 31;282(35):25604-12. doi: 10.1074/jbc.M704343200. Epub 2007 Jun 28. J Biol Chem. 2007. PMID: 17599906 - Autoregulation of the mechanistic target of rapamycin (mTOR) complex 2 integrity is controlled by an ATP-dependent mechanism.
Chen CH, Kiyan V, Zhylkibayev AA, Kazyken D, Bulgakova O, Page KE, Bersimbaev RI, Spooner E, Sarbassov DD. Chen CH, et al. J Biol Chem. 2013 Sep 20;288(38):27019-27030. doi: 10.1074/jbc.M113.498055. Epub 2013 Aug 8. J Biol Chem. 2013. PMID: 23928304 Free PMC article. - Targeted Inhibition of Rictor/mTORC2 in Cancer Treatment: A New Era after Rapamycin.
Zou Z, Chen J, Yang J, Bai X. Zou Z, et al. Curr Cancer Drug Targets. 2016;16(4):288-304. doi: 10.2174/1568009616666151113120830. Curr Cancer Drug Targets. 2016. PMID: 26563881 Review. - Unmasking the tumourigenic role of SIN1/MAPKAP1 in the mTOR complex 2.
Ezine E, Lebbe C, Dumaz N. Ezine E, et al. Clin Transl Med. 2023 Oct;13(10):e1464. doi: 10.1002/ctm2.1464. Clin Transl Med. 2023. PMID: 37877351 Free PMC article. Review.
Cited by
- Exploring Key Challenges of Understanding the Pathogenesis of Kidney Disease in Bardet-Biedl Syndrome.
Marchese E, Ruoppolo M, Perna A, Capasso G, Zacchia M. Marchese E, et al. Kidney Int Rep. 2020 Jun 29;5(9):1403-1415. doi: 10.1016/j.ekir.2020.06.017. eCollection 2020 Sep. Kidney Int Rep. 2020. PMID: 32954066 Free PMC article. Review. - Single-Nucleotide Polymorphisms (SNPs) Both Associated with Hypertension and Contributing to Accelerated-Senescence Traits in OXYS Rats.
Devyatkin VA, Redina OE, Muraleva NA, Kolosova NG. Devyatkin VA, et al. Int J Mol Sci. 2020 May 17;21(10):3542. doi: 10.3390/ijms21103542. Int J Mol Sci. 2020. PMID: 32429546 Free PMC article. - Kinesin 1 regulates cilia length through an interaction with the Bardet-Biedl syndrome related protein CCDC28B.
Novas R, Cardenas-Rodriguez M, Lepanto P, Fabregat M, Rodao M, Fariello MI, Ramos M, Davison C, Casanova G, Alfaya L, Lecumberry F, González-Sapienza G, Irigoín F, Badano JL. Novas R, et al. Sci Rep. 2018 Feb 14;8(1):3019. doi: 10.1038/s41598-018-21329-6. Sci Rep. 2018. PMID: 29445114 Free PMC article. - Left-right asymmetry: lessons from Cancún.
Burdine RD, Caspary T. Burdine RD, et al. Development. 2013 Nov;140(22):4465-70. doi: 10.1242/dev.097907. Development. 2013. PMID: 24194469 Free PMC article. - CCDC28A deficiency causes head-tail coupling defects and immotility in murine spermatozoa.
Stojanovic N, Hernández RO, Ramírez NT, Martínez OME, Hernández AH, Shibuya H. Stojanovic N, et al. Sci Rep. 2024 Nov 5;14(1):26808. doi: 10.1038/s41598-024-78453-9. Sci Rep. 2024. PMID: 39500989 Free PMC article.
References
- Badano J.L., Mitsuma N., Beales P.L., Katsanis N. The Ciliopathies: an emerging class of human genetic disorders. Annu. Rev. Genomics Hum. Genet. 2006;22:125–148. - PubMed
- Fliegauf M., Benzing T., Omran H. When cilia go bad: cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. - PubMed
- Rosenbaum J.L., Witman G.B. Intraflagellar transport. Nat. Rev. Mol. Cell Biol. 2002;3:813–825. - PubMed
- Lopes S.S., Lourenço R., Pacheco L., Moreno N., Kreiling J., Saúde L. Notch signalling regulates left-right asymmetry through ciliary length control. Development. 2010;137:3625–3632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HD04260/HD/NICHD NIH HHS/United States
- DK072301/DK/NIDDK NIH HHS/United States
- R01 DK072301/DK/NIDDK NIH HHS/United States
- R01 DK075972/DK/NIDDK NIH HHS/United States
- R01 HD042601/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous