A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence - PubMed (original) (raw)
Clinical Trial
. 2013 Jul-Aug;7(4):277-86.
doi: 10.1097/ADM.0b013e31829623f4.
Megan L Ryan, Joanne B Fertig, Daniel E Falk, Bankole Johnson, Kelly E Dunn, Alan I Green, Helen M Pettinati, Domenic A Ciraulo, Ofra Sarid-Segal, Kyle Kampman, Mary F Brunette, Eric C Strain, Nassima A Tiouririne, Janet Ransom, Charles Scott, Robert Stout; NCIG (National Institute on Alcohol Abuse and Alcoholism Clinical Investigations Group) Study Group
Affiliations
- PMID: 23728065
- PMCID: PMC3914416
- DOI: 10.1097/ADM.0b013e31829623f4
Clinical Trial
A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence
Raye Z Litten et al. J Addict Med. 2013 Jul-Aug.
Abstract
Objectives: To assess the efficacy and safety of varenicline (Chantix) for the treatment of alcohol dependence. Varenicline is a partial α4β2 nicotinic acetylcholine agonist approved by the Food and Drug Administration for smoking cessation. It has reduced drinking in animal studies and in small studies of humans who were both heavy drinkers and smokers. This is the first multisite clinical trial of varenicline in a population of smokers and nonsmokers with alcohol dependence.
Methods: Men and women (n = 200) meeting the criteria for alcohol dependence were recruited across 5 clinical sites. Patients received double-blind varenicline or placebo and a computerized behavioral intervention. Varenicline was titrated during the first week to 2 mg/d, which was maintained during weeks 2 to 13.
Results: The varenicline group had significantly lower weekly percent heavy drinking days (primary outcome) (adjusted mean difference = 10.4), drinks per day, drinks per drinking day, and alcohol craving compared with the placebo group (P < 0.05). The average treatment effect on alcohol use was similar for smokers and nonsmokers. Varenicline was well-tolerated; adverse events were expected and mild.
Conclusions: Varenicline significantly reduced alcohol consumption and craving, making it a potentially viable option for the treatment of alcohol dependence.
Trial registration: ClinicalTrials.gov NCT01146613.
Figures
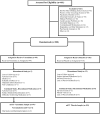
Figure 1. Subject Disposition
mITT = modified intention-to-treat * The mITT sample size of the varenicline group was decreased by n=2 from n=97 to 95 as one patient enrolled twice in the study (at two different study sites), gave invalid data and, consequently, both occurrences of the patient were excluded. The outcome analytic sample size of the varenicline group was further decreased to n=96 as an additional patient discontinued the study prior to reporting outcome data.
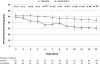
Figure 2. Weekly Differences Between Placebo and Varenicline on the Primary Outcome Measure, Percent Heavy Drinking Days, During Study Maintenance Phase (Weeks 2–13)
* p<.05; ** p<.01 Means are LSMEANS obtained during the maintenance period (Weeks 2-13) from a mixed model that includes treatment group, week, site, treatment goal, craving, baseline percent heavy drinking days, and treatment group by week interaction. Error bars are standard errors. Note: the treatment group by week interaction is statistically significant (p=0.011).
Similar articles
- Varenicline reduces alcohol self-administration in heavy-drinking smokers.
McKee SA, Harrison EL, O'Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. McKee SA, et al. Biol Psychiatry. 2009 Jul 15;66(2):185-90. doi: 10.1016/j.biopsych.2009.01.029. Epub 2009 Feb 27. Biol Psychiatry. 2009. PMID: 19249750 Free PMC article. Clinical Trial. - Varenicline decreases alcohol consumption in heavy-drinking smokers.
Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Mitchell JM, et al. Psychopharmacology (Berl). 2012 Oct;223(3):299-306. doi: 10.1007/s00213-012-2717-x. Epub 2012 May 1. Psychopharmacology (Berl). 2012. PMID: 22547331 Free PMC article. Clinical Trial. - Varenicline treatment of concurrent alcohol and nicotine dependence in schizophrenia: a randomized, placebo-controlled pilot trial.
Meszaros ZS, Abdul-Malak Y, Dimmock JA, Wang D, Ajagbe TO, Batki SL. Meszaros ZS, et al. J Clin Psychopharmacol. 2013 Apr;33(2):243-7. doi: 10.1097/JCP.0b013e3182870551. J Clin Psychopharmacol. 2013. PMID: 23422399 Clinical Trial. - Varenicline: a first-line treatment option for smoking cessation.
Garrison GD, Dugan SE. Garrison GD, et al. Clin Ther. 2009 Mar;31(3):463-91. doi: 10.1016/j.clinthera.2009.03.021. Clin Ther. 2009. PMID: 19393839 Review. - Varenicline in the treatment of alcohol use disorders.
Erwin BL, Slaton RM. Erwin BL, et al. Ann Pharmacother. 2014 Nov;48(11):1445-55. doi: 10.1177/1060028014545806. Epub 2014 Aug 5. Ann Pharmacother. 2014. PMID: 25095786 Review.
Cited by
- Nicotinic receptor modulation to treat alcohol and drug dependence.
Rahman S, Engleman EA, Bell RL. Rahman S, et al. Front Neurosci. 2015 Jan 15;8:426. doi: 10.3389/fnins.2014.00426. eCollection 2014. Front Neurosci. 2015. PMID: 25642160 Free PMC article. Review. - Nicotine receptor partial agonists for smoking cessation.
Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Cahill K, et al. Cochrane Database Syst Rev. 2016 May 9;2016(5):CD006103. doi: 10.1002/14651858.CD006103.pub7. Cochrane Database Syst Rev. 2016. PMID: 27158893 Free PMC article. Updated. Review. - Efficacy of Varenicline in the Treatment of Alcohol Dependence: An Updated Meta-Analysis and Meta-Regression.
Phimarn W, Sakhancord R, Paitoon P, Saramunee K, Sungthong B. Phimarn W, et al. Int J Environ Res Public Health. 2023 Feb 24;20(5):4091. doi: 10.3390/ijerph20054091. Int J Environ Res Public Health. 2023. PMID: 36901103 Free PMC article. Review. - Pharmacological approaches to reducing craving in patients with alcohol use disorders.
Haass-Koffler CL, Leggio L, Kenna GA. Haass-Koffler CL, et al. CNS Drugs. 2014 Apr;28(4):343-60. doi: 10.1007/s40263-014-0149-3. CNS Drugs. 2014. PMID: 24573997 Free PMC article. Review. - Evaluation of guanfacine as a potential medication for alcohol use disorder in long-term drinking rats: behavioral and electrophysiological findings.
Fredriksson I, Jayaram-Lindström N, Wirf M, Nylander E, Nyström E, Jardemark K, Steensland P. Fredriksson I, et al. Neuropsychopharmacology. 2015 Mar 13;40(5):1130-40. doi: 10.1038/npp.2014.294. Neuropsychopharmacology. 2015. PMID: 25359257 Free PMC article.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, D.C.: American Psychiatric Publishing, Inc.; 1994. DSM–IV.
- Blomqvist O, Henandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–31. - PubMed
- Bouchery EE, Henrick MS, Harwood J, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–24. - PubMed
- Cohen J. A power primer. Psychol Bull. 1992;112:155–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical