Expression of murine Unc93b1 is up-regulated by interferon and estrogen signaling: implications for sex bias in the development of autoimmunity - PubMed (original) (raw)
Expression of murine Unc93b1 is up-regulated by interferon and estrogen signaling: implications for sex bias in the development of autoimmunity
Ravichandran Panchanathan et al. Int Immunol. 2013 Sep.
Abstract
The endoplasmic reticulum transmembrane protein, Unc93b1, is essential for trafficking of endosomal TLRs from the endoplasmic reticulum to endosomes. A genetic defect in the human UNC93B1 gene is associated with immunodeficiency. However, systemic lupus erythematosus (SLE) patients express increased levels of the UNC93B1 protein in B cells. Because SLE in patients and certain mouse models exhibits a sex bias and increased serum levels of type I interferons in patients are associated with the disease activity, we investigated whether the female sex hormone estrogen (E2) or type I interferon signaling could up-regulate the expression of the murine Unc93b1 gene. We found that steady-state levels of Unc93b1 mRNA and protein were measurably higher in immune cells (CD3(+), B220(+), CD11b(+) and CD11c(+)) isolated from C57BL/6 (B6) females than age-matched males. Moreover, treatment of CD11b(+) and B220(+) cells with E2 or interferons (IFN-α, IFN-β or IFN-γ) significantly increased the levels of Unc93b1 mRNA and protein. Accordingly, a deficiency of estrogen receptor-α or STAT1 expression in immune cells decreased the expression levels of the Unc93b1 protein. Interestingly, levels of Unc93b1 protein were appreciably higher in B6.Nba2 lupus-prone female mice compared with age-matched B6 females. Furthermore, increased expression of the interferon- and E2-inducible p202 protein in a murine macrophage cell line (RAW264.7) increased the levels of the Unc93b1 protein, whereas knockdown of p202 expression reduced the levels. To our knowledge, our observations demonstrate for the first time that activation of interferon and estrogen signaling in immune cells up-regulates the expression of murine Unc93b1.
Keywords: IFN; SLE; TLRs; Unc93b1; estrogen; innate immune response; p202.
Figures
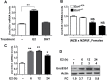
Fig. 1.
Steady-state levels of Unc93b1 mRNA and protein depend on the sex of mice and treatment of immune cells with estrogen or type I interferon increases the levels. (A) Total RNA from the indicated purified immune cells isolated from either C57BL/6 male (n = 4) or age-matched female (n = 4) mice was subjected to quantitative real-time PCR using the TaqMan assays specific to the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the Unc93b1 mRNA to β2-microglobulin mRNA). The ratio of mRNA levels in CD3+ cells from male mice is indicated as 1. The error bars represent the standard deviation (*P < 0.05). (B) Total cell extracts from the indicated purified immune cells isolated from either C57BL/6 males (M; n = 4) or age-matched females (F; n = 4) were subjected to immunoblotting using antibodies specific to the indicated proteins. FC, fold change in the Unc93b1 protein levels is indicated. (C) Purified CD11b+ cells isolated from C57BL/6 male (n = 6) or age-matched female (n = 6) mice were either left untreated (control) or treated with IFN-α (1000U ml−1), 17β-estradiol (E2; 10nM) or DHT (10nM) as described in Methods for 18h. After the treatment, total cell extracts containing approximately equal amounts of proteins were subjected to immunoblotting using antibodies specific to the indicated proteins. FC, fold change in the Unc93b1 protein levels is indicated.
Fig. 2.
Estrogen signaling contributes to increases in Unc93b1 mRNA and protein levels. (A) Purified CD11b+ cells isolated from C57BL/6 female mice (n = 4) were either left untreated or treated with 17β-estradiol (E2; 10nM) or DHT (10nM) as described in Methods for 18h. Total RNA was subjected to quantitative real-time PCR using the TaqMan assay specific to the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the Unc93b1 mRNA to β2-microglobulin mRNA). The ratio of mRNA levels in untreated cells is indicated as 1. The error bars represent the standard deviation (**P < 0.005). (B) Total RNA that was isolated from splenic cells derived from the wild-type (NZB × NZW)F1 or age-matched ERα-deficient (NZB × NZW)F1 females was analyzed by quantitative real-time PCR using the TaqMan assay specific to the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the Unc93b1 mRNA to β2-microglobulin mRNA). The ratio of mRNA levels in one of the wild-type females is indicated as 1. The error bars represent the standard deviation. NS, not significant. (C) Purified CD11b+ cells from C57BL/6 female mice (n = 4) were either left untreated or treated with 17β-estradiol (E2; 10nM) for the indicated time (h). Total RNA was subjected to quantitative real-time PCR using the TaqMan assay specific to the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the Unc93b1 mRNA to β2-microglobulin mRNA). The ratio of mRNA levels in untreated cells is indicated as 1. The error bars represent the standard deviation (*P < 0.05; **P < 0.005). (D) Purified CD11b+ cells from C57BL/6 female mice (n = 4) were either left untreated or treated with 17β-estradiol (E2; 10nM) for the indicated time (h). After the treatment, total cell extracts containing approximately equal amounts of proteins were subjected to immunoblotting using antibodies specific to the indicated proteins. FC, fold change in the Unc93b1 protein levels is indicated.
Fig. 3.
Activation of interferon signaling up-regulates Unc93b1 expression. (A) Purified B220+ cells isolated from C57BL/6 female mice (n = 4) were either left untreated or treated with IFN-α (1000U ml−1), IFN-β (1000U ml−1) or IFN-γ (10ng ml−1) for 6h. Total RNA was subjected to semi-quantitative PCR using a pair of primers that were specific to the murine Unc93b1 gene. (B) RNA samples that were prepared in panel (A) were also subjected to quantitative real-time PCR using the TaqMan assay specific to the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units (one unit being the ratio of the Unc93b1 mRNA to β2-microglobulin mRNA). The ratio of mRNA levels in untreated cells is indicated as 1. The error bars represent the standard deviation (*P < 0.05; **P < 0.005). (C) Purified B220+ cells isolated from C57BL/6 female mice (n = 4) were either left untreated or treated with IFN-α (1000U ml−1), IFN-β (1000U ml−1) or IFN-γ (10ng ml−1) for 6h. Total cell extracts containing approximately equal amounts of proteins were subjected to immunoblotting using antibodies specific to the indicated proteins. FC, fold change in levels of Unc93b1 protein is indicated. (D) Total cell extracts from splenic cells derived from the wild-type or STAT1-deficient male or age-matched female mice (n = 2) were analyzed by immunoblotting using antibodies specific to the indicated proteins. FC, fold change in levels of Unc93b1 protein is indicated. (E) Total RNA from splenic cells derived from the wild-type or STAT1-deficient male or age-matched female mice (n = 2) was analyzed by quantitative real-time PCR using the TaqMan assay specific to the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units. The ratio of mRNA levels in the wild-type males is indicated as 1. The error bars represent the standard deviation (NS, not significant; *P < 0.05).
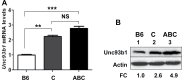
Fig. 4.
Lupus-prone B6.Nba2 female mice express increased levels of Unc93b1. (A) Total RNA isolated from splenic cells derived from age-matched C57BL/6J (B6), B6._Nba2_-C (C) or B6._Nba2_-ABC (ABC) female mice (n = 3 for each strain) was subjected to quantitative real-time PCR using the TaqMan assay specific to the Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units. The ratio of mRNA levels in the B6 females is indicated as 1. The error bars represent the standard deviation (**P < 0.005; ***P < 0.001; NS, not significant). (B) Total cell extracts from splenic cells isolated from age-matched C57BL/6J (B6), B6._Nba2_-C (C) or B6._Nba2_-ABC (ABC) female mice (n = 3) were subjected to immunoblotting using antibodies specific to the indicated proteins. FC, fold change in levels of Unc93b1 protein is indicated.
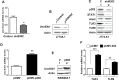
Fig. 5.
p202 protein up-regulates Unc93b1 expression. (A) Total RNA isolated from J774.A1 cells that were stably infected with either control lentivirus (control) or the virus encoding sh_Ifi202_ was subjected to quantitative real-time PCR using TaqMan assay for the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units. The ratio of mRNA levels in the control cells is indicated as 1. The error bars represent the standard deviation (*P < 0.05). (B) Total cell extracts from cells described in panel (A) were subjected to immunoblotting using the antibodies indicated. (C) Total cell extracts from cells described in panel (B) were subjected to immunoblotting using antibodies specific to the indicated proteins. (D) Total RNA isolated from RAW264.7 cells that were nucleofected with either an empty pCMV vector or the pCMV-202 plasmid (encoding the p202 protein) was subjected to qPCR using TaqMan assay for the Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units. The ratio of mRNA levels in the pCMV nucleofected cells is indicated as 1. The error bars represent the standard deviation (**P < 0.005). (E) Total cell extracts from cells described in panel (C) were subjected to immunoblotting using the antibodies indicated. (F) Total RNA as indicated in panel (D) was subjected to qPCR using TaqMan assay for the Tlr3 and Tlr9 genes. The ratio of the TLR3 and TLR9 mRNA levels to β2-microglobulin mRNA was calculated in units. The ratio of mRNA levels in the pCMV nucleofected cells is indicated as 1. The error bars represent the standard deviation (**P < 0.005; ***P < 0.001).
Fig. 6.
Estrogen or IFN-α-mediated up-regulation of Unc93b1 expression depends on p202 protein expression. (A) Murine J774.A1 cells that were stably infected with either control lentivirus or the virus encoding sh_Ifi202_ were either left untreated or treated with IFN-α (IFN, 1000U ml−1) or estrogen (E2, 10nM) for 14h. After the treatments, total RNA was isolated and was subjected to quantitative real-time PCR using TaqMan assay for the murine Unc93b1 gene. The ratio of the Unc93b1 mRNA levels to β2-microglobulin mRNA was calculated in units. The ratio of mRNA levels in the control cells is indicated as 1. The error bars represent the standard deviation (**P < 0.01; ***P < 0.001; NS, not significant). (B) Murine J774.A1 cells that were stably infected with either control lentivirus or the virus encoding sh_Ifi202_ were either left untreated or treated with IFN-α (IFN, 1000U ml−1) or estrogen (E2, 10nM) for 14h. After the treatments, total cell lysates were analyzed by immunoblotting for the indicated proteins. FC, fold change in the Unc93b1 protein levels is indicated.
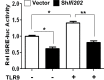
Fig. 7.
Reduced levels of p202 protein decrease TLR9-mediated stimulation of interferon signaling. J774.A1 cells that were stably infected with either control lentivirus or the virus encoding sh_Ifi202_ were transfected with the reporter plasmid ISRE-luc (1.8 μg) along with a second reporter pRL-TK (0.2 μg) using the FuGene 6 transfection agent. Twenty-four hours after transfections, cells were treated with either a control TLR9 ligand or a stimulatory mouse-specific TLR9 ligand (ODN-1668; 5nM) for 6h. At the end of the treatment, cells were lysed and the cell lysates were analyzed for dual luciferase activity. The ratio between the firefly luciferase and the Renilla luciferase in control cells is indicated as 1. The error bars represent the standard deviation. *P < 0.05; **P < 0.005.
Fig. 8.
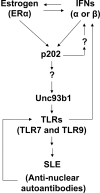
Proposed model for the up-regulation of murine Unc93b1 expression by activation of interferon and estrogen signaling in immune cells.
Similar articles
- Murine BAFF expression is up-regulated by estrogen and interferons: implications for sex bias in the development of autoimmunity.
Panchanathan R, Choubey D. Panchanathan R, et al. Mol Immunol. 2013 Jan;53(1-2):15-23. doi: 10.1016/j.molimm.2012.06.013. Epub 2012 Jul 10. Mol Immunol. 2013. PMID: 22784990 Free PMC article. - Mutually positive regulatory feedback loop between interferons and estrogen receptor-alpha in mice: implications for sex bias in autoimmunity.
Panchanathan R, Shen H, Zhang X, Ho SM, Choubey D. Panchanathan R, et al. PLoS One. 2010 May 28;5(5):e10868. doi: 10.1371/journal.pone.0010868. PLoS One. 2010. PMID: 20526365 Free PMC article. - Bisphenol A (BPA) stimulates the interferon signaling and activates the inflammasome activity in myeloid cells.
Panchanathan R, Liu H, Leung YK, Ho SM, Choubey D. Panchanathan R, et al. Mol Cell Endocrinol. 2015 Nov 5;415:45-55. doi: 10.1016/j.mce.2015.08.003. Epub 2015 Aug 12. Mol Cell Endocrinol. 2015. PMID: 26277401 Free PMC article. - Interferon-inducible Ifi200-family genes as modifiers of lupus susceptibility.
Choubey D. Choubey D. Immunol Lett. 2012 Sep;147(1-2):10-7. doi: 10.1016/j.imlet.2012.07.003. Epub 2012 Jul 24. Immunol Lett. 2012. PMID: 22841963 Free PMC article. Review. - Interferon-inducible Ifi200-family genes in systemic lupus erythematosus.
Choubey D, Panchanathan R. Choubey D, et al. Immunol Lett. 2008 Aug 15;119(1-2):32-41. doi: 10.1016/j.imlet.2008.06.001. Epub 2008 Jul 1. Immunol Lett. 2008. PMID: 18598717 Free PMC article. Review.
Cited by
- Female predisposition to TLR7-driven autoimmunity: gene dosage and the escape from X chromosome inactivation.
Souyris M, Mejía JE, Chaumeil J, Guéry JC. Souyris M, et al. Semin Immunopathol. 2019 Mar;41(2):153-164. doi: 10.1007/s00281-018-0712-y. Epub 2018 Oct 1. Semin Immunopathol. 2019. PMID: 30276444 Review. - Long-Read Sequencing Reveals Rapid Evolution of Immunity- and Cancer-Related Genes in Bats.
Scheben A, Mendivil Ramos O, Kramer M, Goodwin S, Oppenheim S, Becker DJ, Schatz MC, Simmons NB, Siepel A, McCombie WR. Scheben A, et al. Genome Biol Evol. 2023 Sep 4;15(9):evad148. doi: 10.1093/gbe/evad148. Genome Biol Evol. 2023. PMID: 37728212 Free PMC article. - Balance between Estrogens and Proinflammatory Cytokines Regulates Chemokine Production Involved in Thymic Germinal Center Formation.
Dragin N, Nancy P, Villegas J, Roussin R, Le Panse R, Berrih-Aknin S. Dragin N, et al. Sci Rep. 2017 Aug 11;7(1):7970. doi: 10.1038/s41598-017-08631-5. Sci Rep. 2017. PMID: 28801669 Free PMC article. - The Toll for Trafficking: Toll-Like Receptor 7 Delivery to the Endosome.
Petes C, Odoardi N, Gee K. Petes C, et al. Front Immunol. 2017 Sep 4;8:1075. doi: 10.3389/fimmu.2017.01075. eCollection 2017. Front Immunol. 2017. PMID: 28928743 Free PMC article. Review. - Sex bias in autoimmunity.
Billi AC, Kahlenberg JM, Gudjonsson JE. Billi AC, et al. Curr Opin Rheumatol. 2019 Jan;31(1):53-61. doi: 10.1097/BOR.0000000000000564. Curr Opin Rheumatol. 2019. PMID: 30394940 Free PMC article. Review.
References
- Marshak-Rothstein A., Rifkin I. R. 2007. Immunologically active autoantigens: the role of toll-like receptors in the development of chronic inflammatory disease. Annu. Rev. Immunol. 25:419 - PubMed
- Saitoh S., Miyake K. 2009. Regulatory molecules required for nucleotide-sensing Toll-like receptors. Immunol. Rev. 227:32 - PubMed
- Rifkin I. R., Leadbetter E. A., Busconi L., Viglianti G., Marshak-Rothstein A. 2005. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol. Rev. 204:27 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous