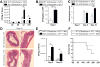Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice - PubMed (original) (raw)
. 2013 Sep;145(3):591-601.e3.
doi: 10.1053/j.gastro.2013.05.047. Epub 2013 May 31.
Kara L Conway, Mei Zhang, Myunghwan Choi, Bret Morin, Zhifang Cao, Eduardo J Villablanca, Chun Li, Cisca Wijmenga, Seok Hyun Yun, Hai Ning Shi, Ramnik J Xavier
Affiliations
- PMID: 23732773
- PMCID: PMC3781941
- DOI: 10.1053/j.gastro.2013.05.047
Card9 mediates intestinal epithelial cell restitution, T-helper 17 responses, and control of bacterial infection in mice
Harry Sokol et al. Gastroenterology. 2013 Sep.
Abstract
Background & aims: Caspase recruitment domain 9 (CARD9) is an adaptor protein that integrates signals downstream of pattern recognition receptors. CARD9 has been associated with autoinflammatory disorders, and loss-of-function mutations have been associated with chronic mucocutaneous candidiasis, but the role of CARD9 in intestinal inflammation is unknown. We characterized the role of Card9 in mucosal immune responses to intestinal epithelial injury and infection.
Methods: We induced intestinal inflammation in Card9-null mice by administration of dextran sulfate sodium (DSS) or Citrobacter rodentium. We analyzed body weight, assessed inflammation by histology, and measured levels of cytokines and chemokines using quantitative reverse-transcription polymerase chain reaction and enzyme-linked immunosorbent assay. Cell populations were compared between wild-type and Card9-null mice by flow cytometry analysis.
Results: Colon tissues and mesenteric lymph nodes of Card9-null mice had reduced levels of interleukin (IL)-6, interferon-γ, and T-helper (Th)17 cytokines after administration of DSS, compared with wild-type mice. IL-17A and IL-22 expression were reduced in the recovery phase after DSS administration, coincident with decreased expression of antimicrobial peptides and the chemokine (C-C motif) ligand 20 (Ccl20). Although Card9-null mice had more intestinal fungi based on 18S analysis, their Th17 responses remained defective even when an antifungal agent was administered throughout DSS exposure. Moreover, Card9-null mice had impaired immune responses to C rodentium, characterized by decreased levels of colonic IL-6, IL-17A, IL-22, and regenerating islet-derived 3 gamma (RegIIIγ), as well as fewer IL-22-producing innate lymphoid cells (ILCs) in colon lamina propria.
Conclusions: The adaptor protein CARD9 coordinates Th17- and innate lymphoid cell-mediated intestinal immune responses after epithelial injury in mice.
Keywords: AMP; ASCA; CARD9; CFU; Ccl20; Colitis; DSS; IFN; IL; ILC; Inflammatory Response; JNK; LP; MLN; Mouse Model; PBS; SFB; T-helper; TNF; Th; antimicrobial peptide; anti–Saccharomyces cerevisiae antibody; c-Jun-N-terminal kinase; caspase recruitment domain 9; chemokine (C-C motif) ligand 20; colony forming unit; dextran sulfate sodium; innate lymphoid cell; interferon; interleukin; lamina propria; mesenteric lymph node; phosphate-buffered saline; qRT-PCR; quantitative reverse-transcription polymerase chain reaction; segmented filamentous bacteria; tumor necrosis factor.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors disclose no conflicts.
Figures
Figure 1
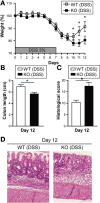
Card9-null mice show impaired recovery after DSS-induced injury. (A) Weight loss in DSS-exposed wild-type (WT) and Card9-null (KO) mice. (B) Colon length of WT and KO mice after administration of DSS. (C) Histologic score of WT and KO mice after administration of DSS. (D) H&E staining of representative cross-sections of medial colon (magnification, 20×). Data are representative of at least 2 independent experiments (N = 5). Error bars represent SEM. *P < .05.
Figure 2
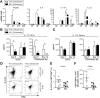
Card9-null mice show impaired immune responses after DSS administration. (A) Cytokine expression in colon of wild-type (WT) and Card9-null (KO) mice as quantified by qRT-PCR. Data are shown as fold changes relative to WT mice without DSS for each time point. (B) Cytokine expression by qRT-PCR (left) and secretion by ELISA (right) in MLN cells after DSS administration and after stimulation with anti-CD3/anti-CD28 antibodies. Data are shown as fold changes relative to WT mice without anti-CD3/anti-CD28. (C) Ccl20 expression in colon of WT and KO mice after DSS administration. Quantification by qRT-PCR. Data are shown as fold changes relative to WT mice without DSS for each time point. (D) Isolated splenic macrophages were stimulated with 100 ng/mL lipopolysaccharide, 10 _μ_g/mL MDP, and 50 μ_g/mL TNF_α for 4 days. Culture supernatants were harvested and analyzed for Ccl20 via enzyme-linked immunosorbent assay. (E) AMP expression in colon of WT and KO mice after recovery from DSS-induced injury. Quantification by qRT-PCR. Data are shown as fold changes relative to WT mice without DSS. (F) Femtosecond laser injuries were introduced into colonic epithelium via a 2-photon endomicroscopy system. Mice were injected with 2 million Daltons fluorescein isothiocyanate dextran before colonoscopy to determine successful photodamage (formation of intravascular clots and extravasation of fluorescent dye after laser exposure). Four days after injury, colons were stained with Evans Blue dye and imaged using bright-field microscopy (top). Residual fluorescein isothiocyanate fluorescence also was assessed using fluorescence microscopy (bottom). Data are representative of at least 2 independent experiments (N = 5). Error bars represent SEM. *P < .05, **P < .01. ND, not detectable.
Figure 3
Card9 is required to control fungi in gut microbiota. (A) Basal fungi levels in fecal microbiota of wild-type (WT) and Card9-null (KO) mice were quantified by 18S qRT-PCR, normalized to the threshold cycle value of the 16S ribosomal RNA gene. Data are plotted relative to WT mice. (B) MLNs were harvested 5 days after DSS was discontinued, and 18S fungi levels were determined by qRT-PCR normalized to glyceraldehyde-3-phosphate dehydrogenase. Data are plotted relative to WT mice. (C) Serum ASCA concentrations were quantified on days 0, 7 (acute injury), and 12 (recovery) via enzyme-linked immunosorbent assay. (D) Weight loss in DSS-treated WT and KO mice. Indicated mice were treated with 0.5 mg/mL fluconazole 2 days before, and throughout the 12-day DSS course described earlier. (E) MLN T cells from fluconazole-treated mice (after acute injury) were stimulated with anti-CD3/anti-CD28 for detection of IFNγ and IL-17A by enzyme-linked immunosorbent assay. Data are representative of at least 2 independent experiments (N = 5–6). Error bars represent SEM. *P < .05.
Figure 4
Card9 is required for Th17 and ILC responses in colon after C rodentium infection. (A) Cytokine expression in colon of wild-type (WT) and Card9-null (KO) mice after C rodentium infection, as quantified by qRT-PCR. Data are shown as fold changes relative to uninfected WT mice. (B–C) Secretion of IL-17A by (B) MLN and (C) spleen cells after C rodentium infection. Cells were stimulated with anti-CD3 or_C rodentium_ antigen. ND, not detectable. (D) IL-17A and IFNγ intracellular staining of CD3+CD4+ T cells from colon LP lymphocytes (top) and MLN (bottom). Representative fluorescence-activated cell sorter plots are shown. (E) Proportion of IL-17A+ cells among colon LP lymphocyte CD3+CD4+ T cells. (F) Number of CD3+CD4+IL-17A+cells in colon LP lymphocytes. Data are representative of at least 2 independent experiments (N = 5). Error bars represent SEM. *P < .05.
Figure 5
Card9 deficiency is associated with decreased ILCs in the gastrointestinal tract during C rodentium infection. (A) Representative fluorescence-activated cell sorter plots of CD3−CD4+IL-17A+ILCs in colon LP cells from wild-type (WT) and Card9-null (KO) mice. (B) Proportion and (C) number of CD3−CD4+IL-17A+ILCs in colon LP. (D) Representative fluorescence-activated cell sorter plots of CD3−CD4−NKP46+ ILCs in colon LP cells. (E) Proportion and (F) number of CD3−CD4−NKP46+ ILCs in colon LP. Data are representative of at least 2 independent experiments (N = 4–6). Error bars represent SEM. *P< .05.
Figure 6
Card9 is required to control C rodentium infection. (A) C rodentium count in feces after infection in wild-type (WT) and Card9-null (KO). (B) C rodentium count in spleen 5 days after infection. (C) Colitis histology score at indicated time points after infection. (D) H&E staining of representative cross-sections of medial colon (magnification, 20x) on day 15 after infection. (E) C rodentium count in feces of mice pretreated with 100 mg nalidixic acid (NA) and infected with 1010 CFU_C rodentium_. (F) Survival curve of mice pretreated with 100 _μ_g NA and infected with 1010 CFU C rodentium. Data are representative of at least 2 independent experiments (N = 5). Error bars represent SEM. *P < .05.
Similar articles
- Role of CARD9 in Cell- and Organ-Specific Immune Responses in Various Infections.
Lee JS, Kim C. Lee JS, et al. Int J Mol Sci. 2024 Feb 23;25(5):2598. doi: 10.3390/ijms25052598. Int J Mol Sci. 2024. PMID: 38473845 Free PMC article. Review. - Effects of hypoxic exposure on immune responses of intestinal mucosa to Citrobacter colitis in mice.
Ji Q, Zhang Y, Zhou Y, Gamah M, Yuan Z, Liu J, Cao C, Gao X, Zhang H, Ren Y, Zhang W. Ji Q, et al. Biomed Pharmacother. 2020 Sep;129:110477. doi: 10.1016/j.biopha.2020.110477. Epub 2020 Jul 6. Biomed Pharmacother. 2020. PMID: 32768962 - Dietary vitamin D3 deficiency alters intestinal mucosal defense and increases susceptibility to Citrobacter rodentium-induced colitis.
Ryz NR, Lochner A, Bhullar K, Ma C, Huang T, Bhinder G, Bosman E, Wu X, Innis SM, Jacobson K, Vallance BA. Ryz NR, et al. Am J Physiol Gastrointest Liver Physiol. 2015 Nov 1;309(9):G730-42. doi: 10.1152/ajpgi.00006.2015. Epub 2015 Sep 3. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 26336925 Free PMC article. - IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation.
Russell SE, Horan RM, Stefanska AM, Carey A, Leon G, Aguilera M, Statovci D, Moran T, Fallon PG, Shanahan F, Brint EK, Melgar S, Hussey S, Walsh PT. Russell SE, et al. Mucosal Immunol. 2016 Sep;9(5):1193-204. doi: 10.1038/mi.2015.134. Epub 2016 Jan 27. Mucosal Immunol. 2016. PMID: 26813344 - CARD9 Regulation and its Role in Cardiovascular Diseases.
Zhang H, Wang Y, Men H, Zhou W, Zhou S, Liu Q, Cai L. Zhang H, et al. Int J Biol Sci. 2022 Jan 1;18(3):970-982. doi: 10.7150/ijbs.65979. eCollection 2022. Int J Biol Sci. 2022. PMID: 35173530 Free PMC article. Review.
Cited by
- Glucocorticoid-Induced Leucine Zipper Protein and Yeast-Extracted Compound Alleviate Colitis and Reduce Fungal Dysbiosis.
Gentili M, Sabbatini S, Nunzi E, Lusenti E, Cari L, Mencacci A, Ballet N, Migliorati G, Riccardi C, Ronchetti S, Monari C. Gentili M, et al. Biomolecules. 2024 Oct 17;14(10):1321. doi: 10.3390/biom14101321. Biomolecules. 2024. PMID: 39456254 Free PMC article. - Fecal microbiota and metabolites in the pathogenesis and precision medicine for inflammatory bowel disease.
Ju L, Suo Z, Lin J, Liu Z. Ju L, et al. Precis Clin Med. 2024 Sep 23;7(3):pbae023. doi: 10.1093/pcmedi/pbae023. eCollection 2024 Sep. Precis Clin Med. 2024. PMID: 39381014 Free PMC article. Review. - Dissecting the respective roles of microbiota and host genetics in the susceptibility of Card9-/- mice to colitis.
Danne C, Lamas B, Lavelle A, Michel ML, Da Costa G, Pham HP, Lefevre A, Bridonneau C, Bredon M, Planchais J, Straube M, Emond P, Langella P, Sokol H. Danne C, et al. Microbiome. 2024 Apr 23;12(1):76. doi: 10.1186/s40168-024-01798-w. Microbiome. 2024. PMID: 38649950 Free PMC article. - Role of CARD9 in Cell- and Organ-Specific Immune Responses in Various Infections.
Lee JS, Kim C. Lee JS, et al. Int J Mol Sci. 2024 Feb 23;25(5):2598. doi: 10.3390/ijms25052598. Int J Mol Sci. 2024. PMID: 38473845 Free PMC article. Review. - Mucosal Immunity to Gut Fungi in Health and Inflammatory Bowel Disease.
Carlson SL, Mathew L, Savage M, Kok K, Lindsay JO, Munro CA, McCarthy NE. Carlson SL, et al. J Fungi (Basel). 2023 Nov 14;9(11):1105. doi: 10.3390/jof9111105. J Fungi (Basel). 2023. PMID: 37998910 Free PMC article. Review.
References
- Ruland J. CARD9 signaling in the innate immune response. Ann N Y Acad Sci. 2008;1143:35–44. - PubMed
- Gross O, Gewies A, Finger K, et al. Card9 controls a non-TLR signalling pathway for innate anti-fungal immunity. Nature. 2006;442:651–656. - PubMed
- Hara H, Ishihara C, Takeuchi A, et al. The adaptor protein CARD9 is essential for the activation of myeloid cells through ITAM-associated and Toll-like receptors. Nat Immunol. 2007;8:619–629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 DK040561/DK/NIDDK NIH HHS/United States
- R01 DK060049/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- DK083756/DK/NIDDK NIH HHS/United States
- DK060049/DK/NIDDK NIH HHS/United States
- DK086502/DK/NIDDK NIH HHS/United States
- R01 DK083756/DK/NIDDK NIH HHS/United States
- DK043351/DK/NIDDK NIH HHS/United States
- RC1 DK086502/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous