Autophagic processes in yeast: mechanism, machinery and regulation - PubMed (original) (raw)
Review
Autophagic processes in yeast: mechanism, machinery and regulation
Fulvio Reggiori et al. Genetics. 2013 Jun.
Abstract
Autophagy refers to a group of processes that involve degradation of cytoplasmic components including cytosol, macromolecular complexes, and organelles, within the vacuole or the lysosome of higher eukaryotes. The various types of autophagy have attracted increasing attention for at least two reasons. First, autophagy provides a compelling example of dynamic rearrangements of subcellular membranes involving issues of protein trafficking and organelle identity, and thus it is fascinating for researchers interested in questions pertinent to basic cell biology. Second, autophagy plays a central role in normal development and cell homeostasis, and, as a result, autophagic dysfunctions are associated with a range of illnesses including cancer, diabetes, myopathies, some types of neurodegeneration, and liver and heart diseases. That said, this review focuses on autophagy in yeast. Many aspects of autophagy are conserved from yeast to human; in particular, this applies to the gene products mediating these pathways as well as some of the signaling cascades regulating it, so that the information we relate is relevant to higher eukaryotes. Indeed, as with many cellular pathways, the initial molecular insights were made possible due to genetic studies in Saccharomyces cerevisiae and other fungi.
Keywords: autophagy; macroautophagy; membrane; organelle; protein degradation; protein trafficking; stress; vacuole.
Figures
Figure 1
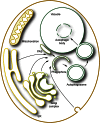
The principal types of autophagy in yeast. Macroautophagy entails the sequestration of bulk cytoplasm or specific structures into autophagomes. Autophagosomes are formed by expansion of a precursor compartment known as the phagophore, which initiates the sequestration of the cargo. Upon completion, the autophagosome fuses with the vacuole, releasing the inner autophagosome vesicle into the vacuole lumen, where it is now termed an autophagic body. During microautophagy (here micropexophagy is illustrated as an example), the structures targeted to degradation are recruited in proximity to the vacuole membrane. Protrusion/septation and/or invagination of this membrane, followed by scission, allows the cargo to be transported into the vacuolar lumen. Via a similar mechanism, micronucleophagy mediates the turnover of part of the nuclear envelope and content. In most cases, the components delivered by macroautophagy, microautophagy, and micronucleophagy into the interior of the vacuole are degraded by resident hydrolases. The resulting metabolites, i.e., amino acids, sugars, and nucleotides, are subsequently transported into the cytoplasm by permeases (although these have been identified only for amino acids) and used either as a source of energy or as building blocks for the synthesis of new macromolecules.
Figure 2
Sequestration of cytoplasmic cargo requires a double-membrane compartment. (A) Exposure of the hydrophobic core of a lipid bilayer to the aqueous cytosol would make it energetically unfavorable to use a single-bilayer membrane to sequester a cytoplasmic cargo. In this scenario, it would also be unclear how the phagophore membrane would expand by lipid addition. (B) The use of a double-lipid bilayer maintains thermodynamic energy requirements, while allowing the cargo to be sequestered by expansion of the double membrane. The expansion of the phagophore could occur by lateral movement or translocation of lipids from an attached organelle, or by vesicular fusion.
Figure 3
Multiple membrane sources may contribute to formation and expansion of the phagophore. Various compartments including the ER, the Golgi apparatus, and the plasma membrane may contribute to the nucleation and/or expansion of the phagophore. See the text for details.
Figure 4
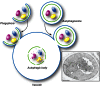
Topology of autophagosome fusion with the vacuole. Fusion of an expanding phagophore (i.e., an incomplete autophagosome) with the vacuole (or lysosome in higher eukaryotes) does not allow delivery of the cytoplasmic content into the interior of the degradative organelle (left side of the drawing). In contrast, the fusion of a sealed autophagosome with the vacuole permits the delivery of its internal vesicle and cargo into the lumen making it accessible for subsequent degradation (right side of the drawing). The mechanism that prevents premature fusion of a phagophore with the vacuole is not known. The electron micrograph shows the presence of autophagic bodies in the vacuole. Scale bar, 1 µm. This image was modified from data previously published in Scott et al. (2000) and is reproduced by permission of the American Society for Biochemistry and Molecular Biology and Elsevier, copyright 2000.
Figure 5
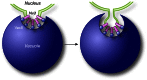
Mechanism of micronucleophagy. During micronucleophagy (also called piecemeal microautophagy of the nucleus), small portions of the nucleus, including the nuclear double membrane and part of the nucleoplasm, protrude into the vacuole lumen though a process that requires the association between Nvj1 in the nuclear membrane and Vac8 on the surface of the vacuole. Subsequently, a scission event mediated by Atg proteins leads to the generation of a subvacuolar vesicle that is degraded by resident hydrolases.
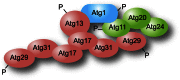
Figure 6
The interactome of the Atg1 complex. Note that there is no indication that all the depicted interactions occur simultaneously, and not all of the known interactions are shown; the Atg1 complex interactors could vary depending on both the step in the formation of the double-membrane vesicle and the type of autophagy.
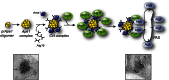
Figure 7
Schematic of the two ubiquitin-like conjugation systems involved in autophagy. Atg12, a ubiquitin-like molecule, is covalently conjugated to Atg5 through the activity of Atg7 and Atg10, an E1- and an E2-like enzyme, respectively. The Atg12—Atg5 complex subsequently associates with Atg16, and dimerization leads to the formation of a large complex. Atg8 is a second ubiquitin-like protein participating in autophagy. Atg8 is post-translationally processed by the specific cysteine protease Atg4, which removes the C-terminal amino acid (an arginine residue in yeast) exposing a glycine. Through another ubiquitination-like reaction mediated by Atg7 and the E2-like enzyme Atg3, Atg8 is covalently conjugated to PE. While it has been proposed that the Atg12—Atg5–Atg16 complex could be the E3 ligase catalyzing the formation of Atg8—PE, these proteins promote the linkage of Atg8 to PE, but they are not essential for it.
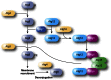
Figure 8
Mechanism of cargo recruitment during the Cvt pathway. Shortly after synthesis, prApe1 forms dodecamers that subsequently self-assemble in a larger oligomer that has been called the prApe1 complex. Association with the Atg19 autophagy receptor and oligomers of Ams1 (and additional cargo proteins) leads to the generation of the Cvt complex. The subsequent interaction between Atg19 and the autophagy adaptor Atg11 allows the movement of the Cvt complex within proximity of the vacuole through a mechanism that requires actin filaments and the Arp2/3 complex. This relocalization, which probably also coordinates the trafficking of Atg9-positive membranes, participates in the formation of the PAS. At this site, the interaction between Atg19 and Atg8 plays a key role in the sequestration of the Cvt complex into Cvt vesicles. One of the primary differences between selective and nonselective macroautophagy is that the sequestering vesicles of the former exclude bulk cytoplasm and contain primarily the targeted cargo. The electron micrographs depict the electron dense Cvt complex detected with antiserum to Ape1 (left) and a phagophore sequestering a Cvt complex marked with an antibody that detects GFP–Atg8 (right). The electron micrographs in this figure were modified from data previously published in Yen et al. (2010) and are reproduced by permission of the American Society for Cell Biology, copyright 2010.
References
- Ano Y., Hattori T., Kato N., Sakai Y., 2005a Intracellular ATP correlates with mode of pexophagy in Pichia pastoris. Biosci. Biotechnol. Biochem. 69: 1527–1533. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases