Embryogenesis of the first circulating endothelial cells - PubMed (original) (raw)
Embryogenesis of the first circulating endothelial cells
Cheng Cui et al. PLoS One. 2013.
Abstract
Prior to this study, the earliest appearance of circulating endothelial cells in warm-blooded animals was unknown. Time-lapse imaging of germ-line transformed Tie1-YFP reporter quail embryos combined with the endothelial marker antibody QH1 provides definitive evidence for the existence of circulating endothelial cells - from the very beginning of blood flow. Blood-smear counts of circulating cells from Tie1-YFP embryos showed that up to 30% of blood-borne cells are Tie1 positive; though cells expressing low levels of YFP were also positive for benzidine, a hemoglobin stain, suggesting that these cells were differentiating into erythroblasts. Electroporation-based time-lapse experiments, exclusively targeting the intra-embryonic mesoderm were combined with QH1 immunostaining. The latter antibody marks quail endothelial cells. Together the optical data provide conclusive evidence that endothelial cells can enter blood flow from vessels of the embryo proper, as well as from extra-embryonic areas. When Tie1-YFP positive cells and tissues are transplanted to wild type host embryos, fluorescent cells emigrate from such transplants and join host vessels; subsequently a few YFP cells are shed into circulation. These data establish that entering circulation is a commonplace activity of embryonic vascular endothelial cells. We conclude that in the class of vertebrates most closely related to mammals a normal component of primary vasculogenesis is production of endothelial cells that enter circulation from all vessels, both intra- and extra-embryonic.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
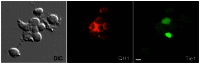
Figure 1. Double fluorescence imaging of adherent cells isolated from embryonic blood.
Cells attached to a culture surface after being harvested from the circulating blood of a HH15 transgenic quail embryo. The green nuclei denote tie1-H2B-YFP expression. The red signal denotes cells reactive with the QH1 antibody. Under the conditions employed, quail erythrocytes (red blood cells) adhered poorly to the culture surface. Differential interference contrast optics (DIC) showed that there are adherent cells present that do not exhibit fluorescence above background levels. Scale bar = 10 µm.
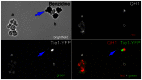
Figure 2. Benzidine staining of circulating whole blood.
Fertilized quail eggs were incubated approximately 50 hours until they had reached between HH15 and HH17. Circulating cells were isolated and stained for QH1 that labels endothelial cells and benzidine that indicates the presence of hemoglobin. Cells expressing low levels of Tie1-YFP (blue arrow) were found to be QH1 negative and benzidine positive, suggesting that the cells were differentiating into erythroblasts. Scale bars = 30 µm.
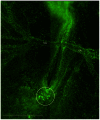
Figure 3. A montage of six image fields showing the vasculature of a Tie1-YFP embryo.
This is a wide-field image frame extracted from a time-lapse recording of the Tie1-YFP transgenic quail embryo shown in Movie S3. Details regarding optics, instrumentation and signal processing are found in Methods and Materials. The fluorescent nuclear (H2B) staining denotes the position of all endothelial cells. The vitelline arteries (VA) are prominent blood vessels at HH15-16. The tail bud mesoderm (circle) shows numerous clusters of primordial endothelial cell nuclei that express the Tie1-YFP marker. Several movies in this report, and recordings not shown, demonstrate that ‘immobile’ clusters of primordial endothelial cells are gradually dispersed by the onset of fluid, flow and subsequently enter circulation. Thus, multi-cellular aggregates are a prominent source of circulating endothelial cells during primary vasculogenesis. The image shown is from Movie S3 at the 16.8 hr time point. Scale bar = 1 mm or 1000 µm.
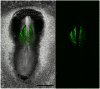
Figure 4. Precise electroporation, ex ovo, allows specific labeling of anterior intra-embryonic mesoderm.
A montage of eight (8) XY microscope fields shows brightfield and epifluorescence images of a HH8 quail embryo that was electroporated approximately 10 hours earlier with Mito-YFP at stage HH4- (see Fig. S1 for details). The left panel shows the two optical modes superimposed. These data demonstrate that using our precision electroporation method it is possible to restrict all Mito-YFP labeling to intra-embryonic tissue exclusively. Specimens that displayed extra-embryonic fluorescence were discarded. Note that the bulk of the fluorescent cells are situated in a mesodermal compartment characterized by robust primary vasculogenesis. Using this targeted approach any Mito-YFP fluorescent cells observed in circulation after HH10 had to have originated from mesoderm of the embryo proper, i.e., did not arise from extra-embryonic mesoderm. Scale bar = 1 mm or 1000 µm.
Figure 5. A sub-population of Mito-GFP labeled intra-embryonic mesoderm differentiates into endothelial cells and expresses the marker QH1.
A DIC time-lapse image frame of a HH9 embryo (panel a), captured during a period of active vasculogenesis. The companion panel (b) shows the corresponding double fluorescence images indicating the presence of primary vascular networks. Red designates QH1 immunoreactivity and green designates Mito-GFP cells labeled by earlier (HH4-) electroporation. A region of interest (white box) is shown at higher magnification in Panel C. Note that at higher magnification there are cells within formed vascular elements that express both red and green fluorescence. Thus, some epiblastic cells electroporated hours earlier had differentiated into vascular endothelial cells at HH9 (QH1, red) when this time-lapse frame was captured. The time-lapse recording from which these images were extracted shows multiple Mito-GFP/QH1-positive cells entering circulation (see Movie S6 and Fig. 6). Note that, as expected, only a sub-population of the Mito-GFP mesoderm (green) differentiated into vascular endothelial cells and react with QH1 endothelial marker (red). Scale bars = 100 µm.
Figure 6. Double fluorescence time-lapse frames of QH1-positive cells and Mito-GFP labeled cells.
All GFP-labeled cells were derived from electroporated intra-embryonic mesoderm shown in Figure 4. This figure is comprised of a series of image frames from a time-lapse recording of the embryo in Movie S6 (i.e., Fig. 5, Fig. 6 and Movie S6 are all the same specimen). Time points and regions of interest (ROI, white boxes) were selected to denote salient features. Each horizontal strip shows five images depicting four time points. The first and second frames in each strip depict the same time point acquired in both the red (QH1) and the green (GFP) optical channels, respectively (e.g., frames 36′ and frame 36). The succeeding three frames in the row show the Mito-GFP signal only. The white arrows denote QH1 positive cells in frame 36′ that correspond to the Mito-GFP positive cells in frame 36. During the time-lapse recording primordial endothelial cells shifted positions and engaged in cell division shown in frames 36-47. For example by frame 41, the second cell from the top (ROI) is condensed and rounded, suggesting possible cell replication, followed by cellular spreading (frames 42, 47). By the time frame 66 was captured all of the Mito-GFP cells in the ROI at frame 52 had moved and/or rounded up. Also, compared to earlier time points fluorescent cells in frame 66 are reduced in number indicating that one or more cells had departed the ROI. After frame 61 the cells of interest continued to move apart, compared to earlier time points; this is denoted by a larger ROI (white box). The GFP-labeled cells visible in frame 70 (circles) are QH1-positive (white boxes with arrows frame 70′). The encircled cell at the bottom of frame 70 is absent from the ROI approximately 13 minutes later (frame 73). In the interval between frames 79 and 80 a cell of interest (circle) was lost from its previous position. The same behavior is observed between frames 84 to 100. The QH1/GFP-positive cells are progressively lost from the ROI, such that by frame 100 all the original cells of interest (frame 36) are absent. Note the consistently bright cells at the periphery of the ROI; this shows that electroporated mesoderm maintains robust Mito-GFP expression throughout the recording. See Methods and Materials, and the Legend for Movie S6 regarding details on optics and image acquisition. Scale bar = 100 µm.
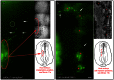
Figure 7. Transplantation of Tie1-YFP tissue or cells into wild type embryos show that donor cells are capable of engaging in vasculogenesis and entering circulation.
The figure shows a single time point selected from two different time-lapse recordings. Both recordings are contained in Movie S11. Panel (a) shows Tie1-YFP tail bud tissue transplanted into a wild-type embryo (n = 6) in which fluorescent cells (green) have emigrated from the explant (red oval) and colonized host vascular elements (white arrows) in 4 out of 6 explants. The white circle indicates a pair of circulating cells that are lost from the ROI in the next frame (see Movie S11a at the 15.46 hr time point). Careful inspection of the Movie S11 reveals other Tie1-YFP endothelial cells that also entered circulation. The red oval in the diagram denotes the initial position of the transplanted tail bud tissue, before recording began. Panel (b) shows a wild-type embryo injected with a bolus of a disperse cell suspension isolated from HH10 Tie1-YFP embryos (n = 4). The image frame shown is extracted from Movie S11b at the 17.46 hr time point. Multiple injections were made parallel to the vertebral axis as denoted by the red stars in the DIC panel and the diagram. Most fluorescent endothelial cells (green) remained near the injection site (red stars). Many YFP cells, however, participated in vessel formation and joined the host vasculature (white arrows). Eventually, a few Tie1-YFP cells entered circulation (see the circled cells in Movie S11b). These data depicting transplanted tissue or cells demonstrate that donor Tie1-YFP endothelial cells are capable of participating in primary vasculogenesis and entering circulation when transferred to a host embryo. The line drawings in Panels 6a and 6b represent an idealized HH7-8 embryo. See the legend for Movie S11 regarding optics and image acquisition. Scale bars = 100 µm.
Similar articles
- Dynamic analysis of vascular morphogenesis using transgenic quail embryos.
Sato Y, Poynter G, Huss D, Filla MB, Czirok A, Rongish BJ, Little CD, Fraser SE, Lansford R. Sato Y, et al. PLoS One. 2010 Sep 14;5(9):e12674. doi: 10.1371/journal.pone.0012674. PLoS One. 2010. PMID: 20856866 Free PMC article. - Extraembryonic origin of circulating endothelial cells.
Pardanaud L, Eichmann A. Pardanaud L, et al. PLoS One. 2011;6(10):e25889. doi: 10.1371/journal.pone.0025889. Epub 2011 Oct 14. PLoS One. 2011. PMID: 22022461 Free PMC article. - Dynamic lineage analysis of embryonic morphogenesis using transgenic quail and 4D multispectral imaging.
Bower DV, Sato Y, Lansford R. Bower DV, et al. Genesis. 2011 Jul;49(7):619-43. doi: 10.1002/dvg.20754. Epub 2011 Jun 17. Genesis. 2011. PMID: 21509927 Review. - Dorsal aorta formation: separate origins, lateral-to-medial migration, and remodeling.
Sato Y. Sato Y. Dev Growth Differ. 2013 Jan;55(1):113-29. doi: 10.1111/dgd.12010. Epub 2012 Nov 8. Dev Growth Differ. 2013. PMID: 23294360 Review.
Cited by
- Comparative analysis of metallic nanoparticles as exogenous soft tissue contrast for live in vivo micro-computed tomography imaging of avian embryonic morphogenesis.
Gregg CL, Butcher JT. Gregg CL, et al. Dev Dyn. 2016 Oct;245(10):1001-10. doi: 10.1002/dvdy.24433. Epub 2016 Aug 18. Dev Dyn. 2016. PMID: 27447729 Free PMC article. - The Early Stages of Heart Development: Insights from Chicken Embryos.
Wittig JG, Münsterberg A. Wittig JG, et al. J Cardiovasc Dev Dis. 2016 Apr 5;3(2):12. doi: 10.3390/jcdd3020012. J Cardiovasc Dev Dis. 2016. PMID: 29367563 Free PMC article. Review. - Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling.
Kasaai B, Caolo V, Peacock HM, Lehoux S, Gomez-Perdiguero E, Luttun A, Jones EA. Kasaai B, et al. Sci Rep. 2017 Mar 8;7:43817. doi: 10.1038/srep43817. Sci Rep. 2017. PMID: 28272478 Free PMC article. - Vessel Enlargement in Development and Pathophysiology.
Gifre-Renom L, Jones EAV. Gifre-Renom L, et al. Front Physiol. 2021 Feb 25;12:639645. doi: 10.3389/fphys.2021.639645. eCollection 2021. Front Physiol. 2021. PMID: 33716786 Free PMC article. Review.
References
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, et al. (2000) Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood 95: 952–958. - PubMed
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967. - PubMed
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, et al. (2002) Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nature medicine 8: 607–612. - PubMed
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, et al. (1999) Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circulation research 85: 221–228. - PubMed
- Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, et al. (2004) Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: final one-year results of the TOPCARE-AMI Trial. Journal of the American College of Cardiology 44: 1690–1699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous