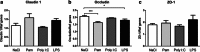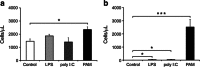Regulation of Toll-like receptors in the choroid plexus in the immature brain after systemic inflammatory stimuli - PubMed (original) (raw)
Regulation of Toll-like receptors in the choroid plexus in the immature brain after systemic inflammatory stimuli
Linnea Stridh et al. Transl Stroke Res. 2013 Apr.
Abstract
The choroid plexus is the site of the blood-cerebrospinal fluid (CSF) barrier (BCSFB) and has also been considered as a possible route for peripheral immune signals and cells to transfer to the central nervous system. Infection/inflammation stimulates innate and subsequent adaptive immune responses via Toll-like receptors (TLRs). In this study, we have investigated the mRNA expression of TLRs, cytokines, and tight junction proteins in the choroid plexus in the immature brain after systemic inflammation, as well as accumulation of immune cells into the CSF. Specific ligands for TLR-1/2, TLR-3, and TLR-4 were administered to postnatal day 8 mice and mRNA expression for the targeted genes was examined in the choroid plexus. We found that mRNA for all four TLRs was detected in the choroid plexus under control conditions. Following immune stimulation, expression of all the TLRs was upregulated by their respective ligands, except for TLR-4 mRNA, which was downregulated by Pam3CSK4 (PAM; a TLR-1/2 ligand). In addition, we investigated BCSFB regulation after TLR stimulation and found that TLR-1/2 and TLR-4 activation was associated with changes in mRNA expression of the tight junction protein occludin in the choroid plexus. PAM induced choroid plexus transcription of TNF-α and resulted in the most dramatic increase in numbers of white blood cells in the CSF. The data suggest a possible mechanism whereby systemic inflammation stimulates TLRs in the choroid plexus, which may lead to disturbances in choroid plexus barrier function, as well as infiltration of immune cells through the plexus.
Keywords: Brain inflammation; Cerebrospinal fluid; LPS; Neonatal; Pam3CSK4; Poly I:C; White blood cells.
Figures
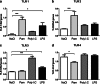
Fig. 1
TLR mRNA regulation in the choroid plexus. TLR-specific ligands were administered and mRNA expression of TLR-1, TLR-2, TLR-3, and TLR-4 in the choroid plexus was examined 14 h postinjection. Bar graphs show the mRNA expression for TLR-1 (a), TLR-2 (b), TLR-3 (c), and TLR-4 (d) after different TLR agonist stimulation. Data are normalized against the geometric mean of the reference genes GAPDH and YWHAZ and shown as the mean ± SEM; *p ≤ 0.05, ***p ≤ 0.001, n = 6/group
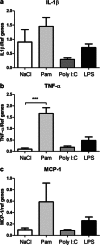
Fig. 2
Regulation of the immune response in the choroid plexus following TLR stimulation. TLR-specific ligands were administered and mRNA expression of the cytokines IL-1β and TNF-α and the chemokine MCP-1 in the choroid plexus was examined 14 h postinjection. The expression of TNF-α was upregulated after PAM (b). No changes were found in IL-1β or MCP-1 expression after the administration of the different ligands (a, c). Data are normalized against the geometric mean of the reference genes GAPDH and YWHAZ and shown as the mean ± SEM; ***p ≤ 0.001, n = 6/group
Fig. 3
Regulation of barrier proteins in the choroid plexus following TLR stimulation. TLR-specific ligands were administered and mRNA expression of several barrier proteins in the choroid plexus was examined 14 h postinjection. The expression of occludin was downregulated after PAM and LPS exposure (b). No changes were found in ZO-1 or claudin-1 expression after the administration of the different ligands (a, c). Data are normalized against the geometric mean of the reference genes GAPDH and YWHAZ and shown as the mean ± SEM; *p ≤ 0.05, ***p ≤ 0.001, n = 5–6/group
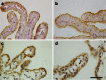
Fig. 4
Tight junction proteins in the choroid plexus following PAM stimulation. Immunoreactivity for the tight junction proteins occludin and ZO-1 in saline-exposed and PAM-exposed animals. Occludin and ZO-1 are present towards the apical side of the epithelial cells of control animals (a, c, respectively) and this staining pattern does not appear to change after PAM treatment (b, c, respectively). Scale bar is 25 μm
Fig. 5
White blood cells after TLR stimulation. The number of white blood cells was counted in the blood (a) and CSF (b) 14 h after the administration of TLR ligands at PND 8. In the blood, there was a significant increase in cell numbers only after TLR-1/2 stimulation (PAM) with around a 60 % increase compared to saline-injected controls. In the CSF, all ligand treatments resulted in significantly increased numbers of cells with around 50 cells/μl after LPS and Poly I:C treatments and 2,500 cells/μl after PAM treatment compared to 8 cells/μl in controls. Data are shown as mean ± SEM; *p ≤ 0.05, ***p ≤ 0.001, n = 5/group
Similar articles
- Choroid plexus transcriptome and ultrastructure analysis reveals a TLR2-specific chemotaxis signature and cytoskeleton remodeling in leukocyte trafficking.
Mottahedin A, Joakim Ek C, Truvé K, Hagberg H, Mallard C. Mottahedin A, et al. Brain Behav Immun. 2019 Jul;79:216-227. doi: 10.1016/j.bbi.2019.02.004. Epub 2019 Feb 26. Brain Behav Immun. 2019. PMID: 30822467 Free PMC article. - Cardiotrophin-1 in choroid plexus and the cerebrospinal fluid circulatory system.
Gard AL, Gavin E, Solodushko V, Pennica D. Gard AL, et al. Neuroscience. 2004;127(1):43-52. doi: 10.1016/j.neuroscience.2004.03.065. Neuroscience. 2004. PMID: 15219667 - Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation.
Marques F, Sousa JC, Coppola G, Falcao AM, Rodrigues AJ, Geschwind DH, Sousa N, Correia-Neves M, Palha JA. Marques F, et al. J Cereb Blood Flow Metab. 2009 May;29(5):921-32. doi: 10.1038/jcbfm.2009.15. Epub 2009 Feb 25. J Cereb Blood Flow Metab. 2009. PMID: 19240744 - The Choroid Plexus in Healthy and Diseased Brain.
Kaur C, Rathnasamy G, Ling EA. Kaur C, et al. J Neuropathol Exp Neurol. 2016 Mar;75(3):198-213. doi: 10.1093/jnen/nlv030. Epub 2016 Feb 17. J Neuropathol Exp Neurol. 2016. PMID: 26888305 Review. - Involvement of the choroid plexus in central nervous system inflammation.
Engelhardt B, Wolburg-Buchholz K, Wolburg H. Engelhardt B, et al. Microsc Res Tech. 2001 Jan 1;52(1):112-29. doi: 10.1002/1097-0029(20010101)52:1<112::AID-JEMT13>3.0.CO;2-5. Microsc Res Tech. 2001. PMID: 11135454 Review.
Cited by
- In vitro investigation of the effect of proinflammatory cytokines on mouse choroid plexus membrane transporters Ncbe and NKCC1.
Johnsen LØ, Friis KA, Damkier HH. Johnsen LØ, et al. Fluids Barriers CNS. 2023 Oct 12;20(1):71. doi: 10.1186/s12987-023-00474-9. Fluids Barriers CNS. 2023. PMID: 37828581 Free PMC article. - New means to assess neonatal inflammatory brain injury.
Jin C, Londono I, Mallard C, Lodygensky GA. Jin C, et al. J Neuroinflammation. 2015 Sep 25;12:180. doi: 10.1186/s12974-015-0397-2. J Neuroinflammation. 2015. PMID: 26407958 Free PMC article. Review. - Brain Barrier Breakdown as a Cause and Consequence of Neuroinflammation in Sepsis.
Danielski LG, Giustina AD, Badawy M, Barichello T, Quevedo J, Dal-Pizzol F, Petronilho F. Danielski LG, et al. Mol Neurobiol. 2018 Feb;55(2):1045-1053. doi: 10.1007/s12035-016-0356-7. Epub 2017 Jan 14. Mol Neurobiol. 2018. PMID: 28092082 Review. - Baicalin Relieves LPS-Induced Lung Inflammation via the NF-κB and MAPK Pathways.
Shen B, Zhang H, Zhu Z, Ling Z, Zeng F, Wang Y, Wang J. Shen B, et al. Molecules. 2023 Feb 16;28(4):1873. doi: 10.3390/molecules28041873. Molecules. 2023. PMID: 36838858 Free PMC article. - The role of inflammation in perinatal brain injury.
Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, Gressens P. Hagberg H, et al. Nat Rev Neurol. 2015 Apr;11(4):192-208. doi: 10.1038/nrneurol.2015.13. Epub 2015 Feb 17. Nat Rev Neurol. 2015. PMID: 25686754 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous