Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors - PubMed (original) (raw)
Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of NMDA receptors
Sha Sha et al. CNS Neurosci Ther. 2013 Sep.
Abstract
Aims: This study investigated the influence of sigma-1 receptor (σ1 R) deficiency on adult neurogenesis.
Methods: We employed 8-week-old male σ1 R knockout (σ1 R(-/-) ) mice to examine the proliferation and differentiation of progenitor cells, and the survival and neurite growth of newborn neurons in hippocampal dentate gyrus (DG).
Results: In comparison with wild-type (WT) littermates, the numbers of 24-h-old BrdU(+) cells and Ki67(+) cells in σ1 R(-/-) mice increased, while the number of 28-day-old BrdU(+) cells decreased without changes in proportion of BrdU(+) /NeuN(+) cells and BrdU(+) /GFAP(+) cells. The neurite density of newborn neurons was slightly reduced in σ1 R(-/-) mice. In DG granular cells, N-methyl-d-aspartate (NMDA)-activated current (INMDA ) and phosphorylation of NMDA receptor (NMDAr) NR2B were reduced in σ1 R(-/-) mice without the alteration of NR2B expression and membrane properties compared to WT mice. The NR2B antagonist abolished the difference in INMDA between σ1 R(-/-) mice and WT mice. The application of NMDAr agonist in σ1 R(-/-) mice prevented the over-proliferation of cells and reduction in newborn neurons, but it had no effects on the hypoplastic neurite. The administration of NMDAr antagonist in WT mice enhanced the cell proliferation and depressed the survival of newborn neurons.
Conclusion: The σ1 R deficiency impairs neurogenesis in DG through down-regulation of NMDArs.
Keywords: Hippocampus; N-methyl-d-aspartate receptor; Neurogenesis; Sigma-1 receptor; γ-Aminobutyric acid.
© 2013 John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
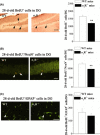
Figure 1
The σ1
R
deficiency enhances the proliferation of progenitor cells in hippocampal dentate gyrus (
DG
). (A) Left panels represent representative images of 24‐h‐old
B
rd
U
- cells (black arrows) in σ1
R
−/− mice and wild‐type (
WT
) mice. Scale bar = 100 μm. Bar graph shows mean number of 24‐h‐old
B
rd
U
- cells in σ1
R
−/− mice (n = 8) and
WT
mice (n = 8). **P <0.01. (B) Representative images of
K
i67+ cells (white arrowheads) in σ1
R
−/− mice and
WT
mice. Scale bar = 50 μm. Bars indicate mean number of
K
i67+ cells in σ1
R
−/− mice (n = 8) and
WT
mice (n = 8). *P <0.05.
B
rd
U
, bromodeoxyuridine.
Figure 2
The σ1
R
deficiency decreases survival of newborn neurons in hippocampal dentate gyrus (
DG
). (A) Left panels represent images of 28‐day‐old
B
rd
U
- cells (black arrows) in σ1
R
−/− mice and wild‐type (
WT
) mice. Scale bar = 100 μm. Bar graph shows mean number of 28‐day‐old
B
rd
U
- cells in σ1
R
−/− mice (n = 8) and
WT
mice (n = 8). **P <0.01. (B, C)
B
rd
U
and
N
eu
N
or
GFAP
were double‐stained on 28th day after injection of
B
rd
U
in σ1
R
−/− mice and
WT
mice (left panel). Neurons (immunoreactive for
N
eu
N
) and glial cells (immunoreactive for
GFAP
) are shown in green, and newborn neurons (immunoreactive for both
B
rd
U
and
N
eu
N
or
GFAP
) are shown in yellow (white arrowheads). Scale bar = 50 μm. Bar graph shows the mean number of 28‐day‐old
B
rd
U
+/
N
eu
N
- cells and
B
rd
U
+/
GFAP
- cells in σ1
R
−/− mice (n = 8) and
WT
mice (n = 8). **P <0.01.
B
rd
U
, bromodeoxyuridine;
GFAP
, antiglial fibrillary acidic protein.
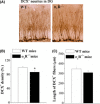
Figure 3
The σ1
R
deficiency decreases neurite growth of newborn neurons in hippocampal dentate gyrus (
DG
). (A) Representative pictures of doublecortin (
DCX
) immunostaining in σ1
R
−/− mice and wild‐type (
WT
) mice. Scale bar = 50 μm. Bars indicate the mean density (B) and length (C) of
DCX
- neurites in σ1
R
−/− mice (n = 8) and
WT
mice (n = 8). *P <0.05.
Figure 4
The σ1
R
deficiency decreases the _N_‐methyl‐
d
‐aspartate receptor (
NMDA
r) function in granular cells of hippocampal dentate gyrus (
DG
). (A) Typical traces of
_I_NMDA
in σ1
R
−/− mice and wild‐type (
WT
) mice (up panel). Bar graph shows mean density of
_I_NMDA
in σ1
R
−/− mice (n = 12) and
WT
mice (n = 12). **P <0.01. (B) Typical traces of
_I_GABA
in σ1
R
−/− mice and
WT
mice (up panel). Bar graph shows mean density of
_I_GABA
in σ1
R
−/− mice (n = 12) and
WT
mice (n = 12). (C) Typical traces of I whole in σ1
R
−/− mice and
WT
mice (up panel). Bar graph shows the maximal amplitudes of outward (
M
axoutward) and inward (
M
axinward) current in σ1
R
−/− mice (n = 12) and
WT
mice (n = 12).
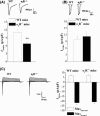
Figure 5
The σ1
R
deficiency reduces phosphorylation of _N_‐methyl‐
d
‐aspartate receptor (
NMDA
r)
NR
2
B
. (A) The levels of NR2A and NR2B m
RNA
in hippocampal dentate gyrus (
DG
) of σ1
R
−/− mice (n = 12) and wild‐type (
WT
) mice (n = 12). (B, C) Western blots of phospho‐
NR
2
A
/2
B
and
NR
2
A
/2
B
in hippocampal
DG
of σ1
R
−/− mice and
WT
mice. The densitometric values for p
NR
2
A
and p
NR
2
B
were first normalized by the protein amounts of
NR
2
A
and
NR
2
B
, respectively, and then were normalized again by the basal values in
WT
mice, respectively. **P <0.01. (D) Bar graph shows mean density of
_I_NMDA
in σ1
R
−/− mice and
WT
mice in the presence of
NR
2
A
antagonist
NVP
‐
AMM
077,
NR
2
B
antagonist
R
o25‐6981, or
SK
channels blocker apamin, respectively (n = 12 mice in each group). **P <0.01; # P <0.05, and ## P <0.01 versus vehicle‐treated slices of
WT
mice or σ1
R
−/− mice.
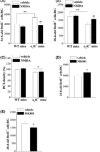
Figure 6
The _N_‐methyl‐
d
‐aspartate receptor (
NMDA
r) agonist corrects the abnormal neurogenesis in σ1
R
−/− mice. (A, B) Bar graphs show mean numbers of 24‐h‐old
B
rd
U
- cells and 28‐day‐old
B
rd
U
- cells in σ1
R
−/− mice or wild‐type (
WT
) mice treated with vehicle or
NMDA
(n = 8 mice in each group). **P <0.01; ## P <0.01 versus vehicle‐treated σ1
R
−/− mice. (C) Bars indicate the density of
DCX
- neurites in σ1
R
−/− mice or
WT
mice treated with
NMDA
(n = 8 mice in each group). *P <0.05 versus vehicle‐treated
WT
mice and σ1
R
−/− mice. (D, E) Bar graphs show the numbers of 24‐h‐old
B
rd
U
- cells and 28‐day‐old
B
rd
U
- cells in
WT
mice treated with vehicle or
MK
801 (n = 8 mice in each group). *P <0.05.
B
rd
U
, bromodeoxyuridine;
DCX
, doublecortin.
Similar articles
- Sex-related neurogenesis decrease in hippocampal dentate gyrus with depressive-like behaviors in sigma-1 receptor knockout mice.
Sha S, Hong J, Qu WJ, Lu ZH, Li L, Yu WF, Chen L. Sha S, et al. Eur Neuropsychopharmacol. 2015 Aug;25(8):1275-86. doi: 10.1016/j.euroneuro.2015.04.021. Epub 2015 May 6. Eur Neuropsychopharmacol. 2015. PMID: 25983018 - Sigma-1 (σ₁) receptor deficiency reduces β-amyloid(25-35)-induced hippocampal neuronal cell death and cognitive deficits through suppressing phosphorylation of the NMDA receptor NR2B.
Yin J, Sha S, Chen T, Wang C, Hong J, Jie P, Zhou R, Li L, Sokabe M, Chen L. Yin J, et al. Neuropharmacology. 2015 Feb;89:215-24. doi: 10.1016/j.neuropharm.2014.09.027. Epub 2014 Oct 5. Neuropharmacology. 2015. PMID: 25286118 - Negative regulation of neurogenesis and spatial memory by NR2B-containing NMDA receptors.
Hu M, Sun YJ, Zhou QG, Chen L, Hu Y, Luo CX, Wu JY, Xu JS, Li LX, Zhu DY. Hu M, et al. J Neurochem. 2008 Aug;106(4):1900-13. doi: 10.1111/j.1471-4159.2008.05554.x. Epub 2008 Jul 9. J Neurochem. 2008. PMID: 18624924 - Effect of the N-methyl-D-aspartate NR2B subunit antagonist ifenprodil on precursor cell proliferation in the hippocampus.
Bunk EC, König HG, Prehn JH, Kirby BP. Bunk EC, et al. J Neurosci Res. 2014 Jun;92(6):679-91. doi: 10.1002/jnr.23347. Epub 2014 Jan 27. J Neurosci Res. 2014. PMID: 24464409 - The role of N-methyl-D-asparate receptors in neurogenesis.
Nacher J, McEwen BS. Nacher J, et al. Hippocampus. 2006;16(3):267-70. doi: 10.1002/hipo.20160. Hippocampus. 2006. PMID: 16425227 Review.
Cited by
- The Sigma Receptors in Alzheimer's Disease: New Potential Targets for Diagnosis and Therapy.
Wang T, Jia H. Wang T, et al. Int J Mol Sci. 2023 Jul 27;24(15):12025. doi: 10.3390/ijms241512025. Int J Mol Sci. 2023. PMID: 37569401 Free PMC article. Review. - Targeting Sigma Receptors for the Treatment of Neurodegenerative and Neurodevelopmental Disorders.
Malar DS, Thitilertdecha P, Ruckvongacheep KS, Brimson S, Tencomnao T, Brimson JM. Malar DS, et al. CNS Drugs. 2023 May;37(5):399-440. doi: 10.1007/s40263-023-01007-6. Epub 2023 May 11. CNS Drugs. 2023. PMID: 37166702 Free PMC article. Review. - Sigma-1 receptor and seizures.
Vavers E, Zvejniece L, Dambrova M. Vavers E, et al. Pharmacol Res. 2023 May;191:106771. doi: 10.1016/j.phrs.2023.106771. Epub 2023 Apr 15. Pharmacol Res. 2023. PMID: 37068533 Free PMC article. Review. - Repeated inhibition of sigma-1 receptor suppresses GABAA receptor expression and long-term depression in the nucleus accumbens leading to depressive-like behaviors.
Qin Y, Xu W, Li K, Luo Q, Chen X, Wang Y, Chen L, Sha S. Qin Y, et al. Front Mol Neurosci. 2022 Sep 30;15:959224. doi: 10.3389/fnmol.2022.959224. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36245919 Free PMC article. - Sigma-1 Receptors in Depression: Mechanism and Therapeutic Development.
Ren P, Wang J, Li N, Li G, Ma H, Zhao Y, Li Y. Ren P, et al. Front Pharmacol. 2022 Jun 16;13:925879. doi: 10.3389/fphar.2022.925879. eCollection 2022. Front Pharmacol. 2022. PMID: 35784746 Free PMC article. Review.
References
- Maurice T, Su TP, Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate β25–35‐amyloid peptide‐induced amnesia in mice through a common mechanism. Neuroscience 1998;83:413–428. - PubMed
- Maurice T. Beneficial effect of the sigma(1) receptor agonist PRE‐084 against the spatial learning deficits in aged rats. Eur J Pharmacol 2001;431:223–227. - PubMed
- Frye CA. The role of neurosteroids and nongenomic effects of progestins in the ventral tegmental area in mediating sexual receptivity of rodents. Horm Behav 2001;40:226–233. - PubMed
- Vallee M, Mayo W, Koob GF, Le Moal M. Neurosteroids in learning and memory processes. Int Rev Neurobiol 2001;46:273–320. - PubMed
- Schumacher M, Weill‐Engerer S, Liere P, et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol 2003;71:3–29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous