The hallmarks of aging - PubMed (original) (raw)
Review
The hallmarks of aging
Carlos López-Otín et al. Cell. 2013.
Abstract
Aging is characterized by a progressive loss of physiological integrity, leading to impaired function and increased vulnerability to death. This deterioration is the primary risk factor for major human pathologies, including cancer, diabetes, cardiovascular disorders, and neurodegenerative diseases. Aging research has experienced an unprecedented advance over recent years, particularly with the discovery that the rate of aging is controlled, at least to some extent, by genetic pathways and biochemical processes conserved in evolution. This Review enumerates nine tentative hallmarks that represent common denominators of aging in different organisms, with special emphasis on mammalian aging. These hallmarks are: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. A major challenge is to dissect the interconnectedness between the candidate hallmarks and their relative contributions to aging, with the final goal of identifying pharmaceutical targets to improve human health during aging, with minimal side effects.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
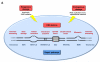
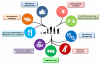
Figure 1. The Hallmarks of Aging
The scheme enumerates the nine hallmarks described in this review: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient-sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication.
Figure 2. Genomic and Epigenomic Alterations
A) Genomic instability and telomere attrition. Endogenous or exogenous agents can stimulate a variety of DNA lesions that are schematically represented on one single chromosome. Such lesions can by repaired by a variety of mechanisms. Excessive DNA damage or insufficient DNA repair favors the aging process. Note that both nuclear DNA and mitochondrial DNA (not represented here) are subjected to age-associated genomic alterations. BER, base excision repair; HR, homologous recombination; NER, nucleotide excision repair; NHEJ, non-homologous end joining; MMR, mismatch repair; ROS, reactive oxygen species; TLS, translesion synthesis; SAC, spindle assembly checkpoint. B) Epigenetic alterations. Alterations in the acetylation and methylation of DNA or histones, as well as of other chromatin-associated proteins, can induce epigenetic changes that contribute to the aging process.
Figure 2. Genomic and Epigenomic Alterations
A) Genomic instability and telomere attrition. Endogenous or exogenous agents can stimulate a variety of DNA lesions that are schematically represented on one single chromosome. Such lesions can by repaired by a variety of mechanisms. Excessive DNA damage or insufficient DNA repair favors the aging process. Note that both nuclear DNA and mitochondrial DNA (not represented here) are subjected to age-associated genomic alterations. BER, base excision repair; HR, homologous recombination; NER, nucleotide excision repair; NHEJ, non-homologous end joining; MMR, mismatch repair; ROS, reactive oxygen species; TLS, translesion synthesis; SAC, spindle assembly checkpoint. B) Epigenetic alterations. Alterations in the acetylation and methylation of DNA or histones, as well as of other chromatin-associated proteins, can induce epigenetic changes that contribute to the aging process.
Figure 3. Loss of Proteostasis
Endogenous and exogenous stress causes the unfolding of proteins (or impairs proper folding during protein synthesis). Unfolded proteins are usually refolded by heat-shock proteins (HSP) or targeted to destruction by the ubiquitin-proteasome or lysosomal (autophagic) pathways. The autophagic pathways include recognition of unfolded proteins by the chaperone Hsc70 and their subsequent import into lysosomes (chaperone-mediated autophagy) or sequestration of damaged proteins and organelles in autophagosomes that later fuse with lysosomes (macroautophagy). Failure to refold or degrade unfolded proteins can lead to their accumulation and aggregation, resulting in proteotoxic effects.
Figure 4. Metabolic Alterations
A) Deregulated nutrient-sensing. Overview of the somatroph axis involving growth hormone (GH) and the insulin/insulin growth factor 1 (IGF-1) signaling pathway, and its relationship to dietary restriction and aging. Molecules that favor aging are shown in orange, while molecules with anti-aging properties are shown in light green. B) Mitochondrial dysfunction. Mitochondrial function becomes perturbed by aging-associated mtDNA mutations, reduced mitochondriogenesis, destabilization of the electron transport chain (ETC) complexes, altered mitochondrial dynamics or defective quality control by mitophagy. Stress signals and defective mitochondrial function generate ROS that, below a certain threshold, induce survival signals to restore cellular homeostasis, but at higher or continued levels can contribute to aging. Similarly, mild mitochondrial damage can induce a hormetic response (mitohormesis) that triggers adaptive compensatory processes.
Figure 4. Metabolic Alterations
A) Deregulated nutrient-sensing. Overview of the somatroph axis involving growth hormone (GH) and the insulin/insulin growth factor 1 (IGF-1) signaling pathway, and its relationship to dietary restriction and aging. Molecules that favor aging are shown in orange, while molecules with anti-aging properties are shown in light green. B) Mitochondrial dysfunction. Mitochondrial function becomes perturbed by aging-associated mtDNA mutations, reduced mitochondriogenesis, destabilization of the electron transport chain (ETC) complexes, altered mitochondrial dynamics or defective quality control by mitophagy. Stress signals and defective mitochondrial function generate ROS that, below a certain threshold, induce survival signals to restore cellular homeostasis, but at higher or continued levels can contribute to aging. Similarly, mild mitochondrial damage can induce a hormetic response (mitohormesis) that triggers adaptive compensatory processes.
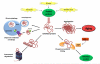
Figure 5. Cellular Senescence, Stem Cell Exhaustion and Altered Intercellular Communication
A) Cellular senescence. In young organisms, cellular senescence prevents the proliferation of damaged cells, thus protecting from cancer and contributing to tissue homeostasis. In old organisms, the pervasive damage and the deficient clearance and replenishment of senescent cells results in their accumulation, and this has a number of deleterious effects on tissue homeostasis that contribute to aging. B) Stem cell exhaustion. Consequences of the exhaustion of hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), satellite cells and intestinal epithelial stem cells (IESCs) are exemplified. C) Altered intercellular communication. Examples of altered intercellular communication associated with aging.
Figure 5. Cellular Senescence, Stem Cell Exhaustion and Altered Intercellular Communication
A) Cellular senescence. In young organisms, cellular senescence prevents the proliferation of damaged cells, thus protecting from cancer and contributing to tissue homeostasis. In old organisms, the pervasive damage and the deficient clearance and replenishment of senescent cells results in their accumulation, and this has a number of deleterious effects on tissue homeostasis that contribute to aging. B) Stem cell exhaustion. Consequences of the exhaustion of hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), satellite cells and intestinal epithelial stem cells (IESCs) are exemplified. C) Altered intercellular communication. Examples of altered intercellular communication associated with aging.
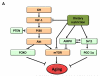
Figure 6. Functional Interconnections between the Hallmarks of Aging
The proposed nine hallmarks of aging are grouped into three categories. In the top, those hallmarks considered to be the primary causes of cellular damage. In the middle, those considered to be part of compensatory or antagonistic responses to the damage. These responses initially mitigate the damage, but eventually, if chronic or exacerbated, they become deleterious themselves. In the bottom, there are integrative hallmarks that are the end result of the previous two groups of hallmarks and are ultimately responsible for the functional decline associated with aging.
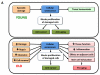
Figure 7. Interventions that Might Extend Human Healthspan
The nine hallmarks of aging are shown together with those therapeutic strategies for which there are proof of principle in mice.
Comment in
- Prelamin A and Oct-1: a puzzle of aging.
Infante A, Rodríguez CI. Infante A, et al. Oncotarget. 2015 Feb 28;6(6):3475-6. doi: 10.18632/oncotarget.3422. Oncotarget. 2015. PMID: 25708685 Free PMC article. No abstract available.
Similar articles
- Hallmarks of aging: An expanding universe.
López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. López-Otín C, et al. Cell. 2023 Jan 19;186(2):243-278. doi: 10.1016/j.cell.2022.11.001. Epub 2023 Jan 3. Cell. 2023. PMID: 36599349 Review. - Mechanistic links between aging and lung fibrosis.
Thannickal VJ. Thannickal VJ. Biogerontology. 2013 Dec;14(6):609-15. doi: 10.1007/s10522-013-9451-6. Epub 2013 Aug 9. Biogerontology. 2013. PMID: 23929205 Free PMC article. Review. - A revisiting of "the hallmarks of aging" in domestic dogs: current status of the literature.
Jiménez AG. Jiménez AG. Geroscience. 2024 Feb;46(1):241-255. doi: 10.1007/s11357-023-00911-5. Epub 2023 Aug 18. Geroscience. 2024. PMID: 37594598 Free PMC article. Review. - The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging.
Farr JN, Almeida M. Farr JN, et al. J Bone Miner Res. 2018 Sep;33(9):1568-1584. doi: 10.1002/jbmr.3564. Epub 2018 Aug 13. J Bone Miner Res. 2018. PMID: 30075061 Free PMC article. Review. - Long noncoding RNAs(lncRNAs) and the molecular hallmarks of aging.
Grammatikakis I, Panda AC, Abdelmohsen K, Gorospe M. Grammatikakis I, et al. Aging (Albany NY). 2014 Dec;6(12):992-1009. doi: 10.18632/aging.100710. Aging (Albany NY). 2014. PMID: 25543668 Free PMC article. Review.
Cited by
- Molecular mechanism of skeletal muscle loss and its prevention by natural resources.
Kim JT, Jeon DH, Lee HJ. Kim JT, et al. Food Sci Biotechnol. 2024 Aug 16;33(15):3387-3400. doi: 10.1007/s10068-024-01678-x. eCollection 2024 Dec. Food Sci Biotechnol. 2024. PMID: 39493391 Review. - Translational control of gene expression by eIF2 modulates proteostasis and extends lifespan.
Jiménez-Saucedo T, Berlanga JJ, Rodríguez-Gabriel M. Jiménez-Saucedo T, et al. Aging (Albany NY). 2021 Apr 26;13(8):10989-11009. doi: 10.18632/aging.203018. Epub 2021 Apr 26. Aging (Albany NY). 2021. PMID: 33901016 Free PMC article. - Modulation of the ubiquitin-proteasome system by marine natural products.
Vasilopoulou MΑ, Ioannou E, Roussis V, Chondrogianni N. Vasilopoulou MΑ, et al. Redox Biol. 2021 May;41:101897. doi: 10.1016/j.redox.2021.101897. Epub 2021 Feb 17. Redox Biol. 2021. PMID: 33640701 Free PMC article. Review. - Centenarians consistently present a younger epigenetic age than their chronological age with four epigenetic clocks based on a small number of CpG sites.
Daunay A, Hardy LM, Bouyacoub Y, Sahbatou M, Touvier M, Blanché H, Deleuze JF, How-Kit A. Daunay A, et al. Aging (Albany NY). 2022 Oct 3;14(19):7718-7733. doi: 10.18632/aging.204316. Epub 2022 Oct 3. Aging (Albany NY). 2022. PMID: 36202132 Free PMC article. - The unfolded protein response reverses the effects of glucose on lifespan in chemically-sterilized C. elegans.
Beaudoin-Chabot C, Wang L, Celik C, Abdul Khalid AT, Thalappilly S, Xu S, Koh JH, Lim VWX, Low AD, Thibault G. Beaudoin-Chabot C, et al. Nat Commun. 2022 Oct 19;13(1):5889. doi: 10.1038/s41467-022-33630-0. Nat Commun. 2022. PMID: 36261415 Free PMC article.
References
- Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, Forsstrom S, Salven P, Angers-Loustau A, Kopra OH, et al. Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab. 2012;15:100–109. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical