Nicotine potentiates proatherogenic effects of oxLDL by stimulating and upregulating macrophage CD36 signaling - PubMed (original) (raw)
Nicotine potentiates proatherogenic effects of oxLDL by stimulating and upregulating macrophage CD36 signaling
Ming-Sheng Zhou et al. Am J Physiol Heart Circ Physiol. 2013.
Abstract
Cigarette smoking is a major risk factor for atherosclerosis and cardiovascular disease. CD36 mediates oxidized LDL (oxLDL) uptake and contributes to macrophage foam cell formation. We investigated a role for the CD36 pathway in nicotine-induced activation of macrophages and foam cell formation in vitro and in vivo. Nicotine in the same plasma concentration range found in smokers increased the CD36(+)/CD14(+) cell population in human peripheral blood mononuclear cells, increased CD36 expression of human THP1 macrophages, and increased macrophage production of reactive oxygen species, PKCδ phosphorylation, and peroxisome proliferator-activated receptor-γ (PPARγ) expression. Nicotine-induced CD36 expression was suppressed by antioxidants and by specific PKCδ and PPARγ inhibitors, implicating mechanistic roles for these intermediates. Nicotine synergized with oxLDL to increase macrophage expression of CD36 and cytokines TNF-α, monocyte chemoattractant protein-1, IL-6, and CXCL9, all of which were prevented by CD36 small interfering (si)RNA. Incubation with oxLDL (50 μg/ml) for 72 h resulted in lipid deposition in macrophages and foam cell formation. Preincubation with nicotine further increased oxLDL-induced lipid accumulation and foam cell formation, which was also prevented by CD36 siRNA. Treatment of apoE-/- mice with nicotine markedly exacerbated inflammatory monocyte levels and atherosclerotic plaque accumulation, effects that were not seen in CD36-/- apoE-/- mice. Our results show that physiological levels of nicotine increase CD36 expression in macrophages, a pathway that may account at least in part for the known proinflammatory and proatherogenic properties of nicotine. These results identify such enhanced CD36 expression as a novel nicotine-mediated pathway that may constitute an independent risk factor for atherosclerosis in smokers. The results also suggest that exacerbated atherogenesis by this pathway may be an adverse side effect of extended use of high concentrations of nicotine independent of their mode of administration.
Keywords: CD36; foam cell; inflammatory cytokine; macrophage; nicotine.
Figures
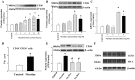
Fig. 1.
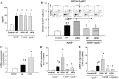
Effect of nicotine on CD36 expression in human THP1 macrophages or isolated human peripheral blood mononuclear cells (PBMCs). A: time course of CD36 protein expression in cells treated with 100 nmol/l nicotine. Nicotine increased protein (B) and mRNA (C) expression of CD36 in a dose-dependent manner. D: nicotine increased cell surface protein expression of CD36 in human PBMCs. Isolated human PBMCs were incubated with 10 nmol/l nicotine for 24 h. Fluorescence-activated cell sorting (FACS) was used to identify cells expressing CD36 (CD36+CD14+ population) in human monocytes (CD14+). E: nonselective nAchR blocker, hexamethonium (100 μmol/l), or an α-7 nAchR blocker, bungarotoxin (1 nmol/l), prevented nicotine-induced CD36 expression. F: nicotine did not affect protein expression of lectin-like oxidized low-density lipoprotein receptor 1 (LOX1) and scavenger receptor (SR) A. Data are expressed as means ± SE. *P < 0.05 vs. control; #P < 0.05 vs. nicotine; n = 5–6.
Fig. 2.
Nicotine (100 nmol/l) increased reactive oxygen species (ROS) production (A_–_C) and PKCδ phosphorylation (D) in THP1 macrophages. ROS was determined by dichlorofluorescein diacetate (DCFDA) assay (A and B) or by lucigenin chemiluminescence (C). Compared with control (a), incubation with nicotine (b) for 15 min significantly increased ROS production, which was inhibited by preincubation with either the nonselective nAchR blocker hexamethonium (100 nmol/l; c), the a-7 nAchR blocker bungarotoxin (1 nmol/l; d), or the NADPH oxidase inhibitor DPI (10 μmol/l). Nicotine-induced ROS production was also blunted (e). Nicotine increased PKCδ phosphorylation (D), and the antioxidant NADPH oxidase inhibitors DPI (10 μmol/l), apocynin (100 μmol/l), or free radical scavenger _N_-acetylcysteine (NAC; 1 mmol/l) significantly inhibited nicotine-induced PKCδ phosphorylation (E). *P < 0.05 vs. control; #P < 0.05 vs. nicotine; n = 4–6.
Fig. 3.
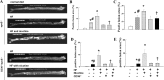
Effects of antioxidants (A), PKC (B), or proliferator-activated receptor-γ (PPARγ) inhibitor (D) on nicotine-induced CD36 expression in human THP1 macrophages. Antioxidant treatment (DPI, apocynin, or NAC) or suppression of PKC by the classic PKC inhibitor calphostin C (0.5 μmol/l) or specific PKCδ inhibitor rottlerin (3 μmol/l) significantly reduced nicotine-induced CD36 expression. Nicotine (100 nmol/l) increased PPARγ expression in a time-dependent manner (C), and inhibition of PPARγ by T0070907 (100 nmol/l) significantly reduced nicotine-induced CD36 expression (D). *P < 0.05 vs. control; #P < 0.05 vs. nicotine; n = 5–6.
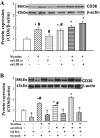
Fig. 4.
Synergistic effects of nicotine and oxidized LDL (oxLDL) on protein expression of CD36 in human THP1 macrophages. A: nicotine and oxLDL at a high dose (50 μg/ml) but not at a low dose (10 μg/ml) increased protein expression of CD36. The combination of nicotine with high- dose oxLDL further increased CD36 expression. B: CD36 small interfering (si)RNA reduced CD36 protein expression. *P < 0.05 vs. control; #P < 0.05 vs. corresponding combination group; †P < 0.05 vs. the corresponding scrambled group; n = 5–6.
Fig. 5.
Nicotine increased oxLDL uptake (A) in human THP1 macrophages. B: CD36 siRNA prevented the increase in nicotine-induced oxLDL uptake. oxLDL uptake was measured by cell-mediated oxLDL degradation assay. Nicotine increased intracellular cholesterol content (C) and promoted oxLDL-induced foam cell formation (D): cells were incubated with oxLDL (50 μg/ml) for 72 h (a); cells were incubated with nicotine (100 nmol/l) for 24 h followed by incubation with oxLDL for 72 h (b); and cells were transfected with the CD36 siRNA and then incubated with nicotine and oxLDL (c). *P < 0.05 vs. control; #P < 0.05 vs. nicotine; n = 5–7.
Fig. 6.
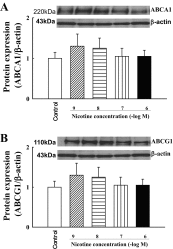
Effects of nicotine (100 nmol/l) on protein expression of the reverse cholesterol transporters ABCA1 (A) and ABCG1 (B) in human THP1/macrophages. Nicotine did not affect ABCA1 and ABCG1 expression; n = 4.
Fig. 7.
Effects of nicotine and/or oxLDL on mRNA expression of inflammatory cytokines in human THP1 macrophages. Nicotine (100 nmol/l) increased mRNA expression of CD36 (A) but did not significantly increase mRNA expression of inflammatory cytokines TNF-α (B), IL-6 (C), monocyte chemoattractant protein 1 (MCP-1; D), and CXCL9 (E). oxLDL (50 μg/ml) increased mRNA expression of CD36 and proinflammatory cytokines (198–377%). The combination of nicotine and oxLDL increased expression of CD36 and inflammatory cytokines (345–712%), which was significantly reduced by the CD36 siRNA. *P < 0.05 vs. control; †P < 0.05 vs. combination group; n = 5.
Fig. 8.
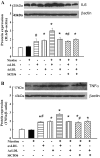
Effects of nicotine, oxLDL, AcLDL, or CD36 siRNA on protein expression of IL-6 (A) and TNF-α (B) in human THP1/macrophages. Nicotine alone did not affect IL-6 and TNF-α expression. AcLDL (50 μg/ml) increased protein expression of IL-6 and TNF-α to the same extent as oxLDL (50 μg/ml). The combination of nicotine and oxLDL, but not AcLDL, further increased expression of IL-6 and TNF-α. CD36 siRNA prevented nicotine potentiation of oxLDL-induced IL-6 and TNF-α expression. *P < 0.05 vs. control; #P < 0.05 vs. corresponding combination group without siRNA; n = 5–6.
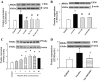
Fig. 9.
Effects of long term treatment with nicotine (100 mg/l in drinking water for 15 wk) on plasma levels of cholesterol (A), the inflammatory monocyte subset (B; CD11b+/Ly6Chi), or mRNA expression of CD36 (C), IL-1β (D), and TNF-α (E) in isolated peritoneal macrophages from apoE−/− or CD36−/− apoE−/− mice. Nicotine significantly increased the circulatory inflammatory monocyte subset (CD11b+/Ly6Chi) and mRNA expression of CD36 and inflammatory cytokines IL-1β and TNF-α in apoE−/− mice fed a high-fat diet. Deletion of the CD36 gene in apoE −/− mice (CD36−/− apoE−/−) prevented nicotine-induced inflammatory monocyte and inflammatory cytokine expression in peritoneal macrophages. *P < 0.05 vs. control; #P < 0.05 vs. nicotine-treated animals; †P < 0.05 vs. corresponding apoE−/− mice; n = 4–6.
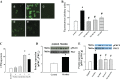
Fig. 10.
Morphometric analysis of lesion area in the aortic tree. Lesion positive areas were identified on the digital image by Ed Herderick, who was blinded to the experimental groups. Proportional lesion areas were calculated by the ratio of positive lesion areas to total (or segmental) area. Nicotine promoted atherosclerotic lesions in apoE −/− mice fed a high-fat, high-cholesterol diet (HF), but these effects were prevented in CD36−/−apoE−/− mice. A: representative photographs of en face aortas from apoE−/−; CD36−/− apoE−/− mice; summarization of lesion area in whole aortas (B), aortic arch (C), thoracic aortas (D), and abdominal aortas (E). *P < 0.05 vs. apoE−/− mice on a normal diet; #P < 0.05 vs. nicotine-treated animals; †P < 0.05 vs. corresponding apoE−/− mice; n = 4–6.
Similar articles
- PKCδ signalling regulates SR-A and CD36 expression and foam cell formation.
Lin CS, Lin FY, Ho LJ, Tsai CS, Cheng SM, Wu WL, Huang CY, Lian CH, Yang SP, Lai JH. Lin CS, et al. Cardiovasc Res. 2012 Aug 1;95(3):346-55. doi: 10.1093/cvr/cvs189. Epub 2012 Jun 11. Cardiovasc Res. 2012. PMID: 22687273 - Trimethylamine N-oxide promotes atherosclerosis via CD36-dependent MAPK/JNK pathway.
Geng J, Yang C, Wang B, Zhang X, Hu T, Gu Y, Li J. Geng J, et al. Biomed Pharmacother. 2018 Jan;97:941-947. doi: 10.1016/j.biopha.2017.11.016. Epub 2017 Nov 7. Biomed Pharmacother. 2018. PMID: 29136772 - Inhibition of Macrophage CD36 Expression and Cellular Oxidized Low Density Lipoprotein (oxLDL) Accumulation by Tamoxifen: A PEROXISOME PROLIFERATOR-ACTIVATED RECEPTOR (PPAR)γ-DEPENDENT MECHANISM.
Yu M, Jiang M, Chen Y, Zhang S, Zhang W, Yang X, Li X, Li Y, Duan S, Han J, Duan Y. Yu M, et al. J Biol Chem. 2016 Aug 12;291(33):16977-89. doi: 10.1074/jbc.M116.740092. Epub 2016 Jun 29. J Biol Chem. 2016. PMID: 27358406 Free PMC article. - Foam cells in atherosclerosis.
Yu XH, Fu YC, Zhang DW, Yin K, Tang CK. Yu XH, et al. Clin Chim Acta. 2013 Sep 23;424:245-52. doi: 10.1016/j.cca.2013.06.006. Epub 2013 Jun 16. Clin Chim Acta. 2013. PMID: 23782937 Review. - CD36, a scavenger receptor implicated in atherosclerosis.
Park YM. Park YM. Exp Mol Med. 2014 Jun 6;46(6):e99. doi: 10.1038/emm.2014.38. Exp Mol Med. 2014. PMID: 24903227 Free PMC article. Review.
Cited by
- Immune-metabolic mechanisms of post-traumatic stress disorder and atherosclerosis.
Tian Y, Ullah H, Gu J, Li K. Tian Y, et al. Front Physiol. 2023 Feb 8;14:1123692. doi: 10.3389/fphys.2023.1123692. eCollection 2023. Front Physiol. 2023. PMID: 36846337 Free PMC article. Review. - New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis.
Wu MY, Li CJ, Hou MF, Chu PY. Wu MY, et al. Int J Mol Sci. 2017 Sep 22;18(10):2034. doi: 10.3390/ijms18102034. Int J Mol Sci. 2017. PMID: 28937652 Free PMC article. Review. - Integrated miRNA-mRNA analysis in the habenula nuclei of mice intravenously self-administering nicotine.
Lee S, Woo J, Kim YS, Im HI. Lee S, et al. Sci Rep. 2015 Aug 11;5:12909. doi: 10.1038/srep12909. Sci Rep. 2015. PMID: 26260614 Free PMC article. - Cardiac macrophages in maintaining heart homeostasis and regulating ventricular remodeling of heart diseases.
Kang M, Jia H, Feng M, Ren H, Gao J, Liu Y, Zhang L, Zhou MS. Kang M, et al. Front Immunol. 2024 Sep 20;15:1467089. doi: 10.3389/fimmu.2024.1467089. eCollection 2024. Front Immunol. 2024. PMID: 39372400 Free PMC article. Review. - Smoking patterns and chronic kidney disease in US Hispanics: Hispanic Community Health Study/Study of Latinos.
Franceschini N, Deng Y, Flessner MF, Eckfeldt JH, Kramer HJ, Lash JP, Lee DJ, Melamed ML, Moncrieft AE, Ricardo AC, Rosas SE, Kaplan RC, Raij L, Cai J. Franceschini N, et al. Nephrol Dial Transplant. 2016 Oct;31(10):1670-6. doi: 10.1093/ndt/gfw210. Epub 2016 Jun 2. Nephrol Dial Transplant. 2016. PMID: 27257272 Free PMC article.
References
- Chelland Campbell S, Moffatt RJ, Stamford BA. Smoking and smoking cessation–the relationship between cardiovascular disease and lipoprotein metabolism: a review. Atherosclerosis 201: 225–235, 2008 - PubMed
- Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc Res 75: 468–477, 2007 - PubMed
- Cornhill JF, Barrett WA, Herderick EE, Mahley RW, Fry DL. Topographic study of sudanophilic lesions in cholesterol-fed minipigs by image analysis. Atherosclerosis 5: 415–426, 1985 - PubMed
- de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: a multifunctional receptor in atherosclerosis. Atheroscler Thromb Vasc Biol 20: 290–297, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL046403/HL/NHLBI NIH HHS/United States
- HL-072924/HL/NHLBI NIH HHS/United States
- R01 HL072924/HL/NHLBI NIH HHS/United States
- NIEHS-014948/PHS HHS/United States
- R01 HL111614/HL/NHLBI NIH HHS/United States
- NIDDK-069372/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous