Lepr(db/db) Mice with senescence marker protein-30 knockout (Lepr(db/db)Smp30(Y/-)) exhibit increases in small dense-LDL and severe fatty liver despite being fed a standard diet - PubMed (original) (raw)
. 2013 Jun 3;8(6):e65698.
doi: 10.1371/journal.pone.0065698. Print 2013.
Goji Hasegawa, Hiroshi Okada, Takafumi Senmaru, Michiaki Fukui, Naoto Nakamura, Morio Sawada, Jo Kitawaki, Takeshi Okanoue, Yuki Kishimoto, Akiko Amano, Naoki Maruyama, Hiroshi Obayashi, Akihito Ishigami
Affiliations
- PMID: 23755269
- PMCID: PMC3670834
- DOI: 10.1371/journal.pone.0065698
Lepr(db/db) Mice with senescence marker protein-30 knockout (Lepr(db/db)Smp30(Y/-)) exhibit increases in small dense-LDL and severe fatty liver despite being fed a standard diet
Yoshitaka Kondo et al. PLoS One. 2013.
Abstract
Background/aims: The senescence marker protein-30 (SMP30) is a 34 kDa protein originally identified in rat liver that shows decreased levels with age. Several functional studies using SMP30 knockout (Smp30(Y/-) ) mice established that SMP30 functions as an antioxidant and protects against apoptosis. To address the potential role of SMP30 in nonalcoholic fatty liver disease (NAFLD) pathogenesis, we established Smp30(Y/-) mice on a Lepr(db/db) background (Lepr(db/db)Smp30(Y/-) mice). RESEARCH DESIGN/PRINCIPAL FINDINGS: Male Lepr(db/db)Smp30(Y/-) mice were fed a standard diet (340 kcal/100 g, fat 5.6%) for 16 weeks whereupon the lipid/lipoprotein profiles, hepatic expression of genes related to lipid metabolism and endoplasmic reticulum stress markers were analyzed by HPLC, quantitative RT-PCR and western blotting, respectively. Changes in the liver at a histological level were also investigated. The amount of SMP30 mRNA and protein in livers was decreased in Lepr(db/db)Smp30(Y/+) mice compared with Lepr(db/+)Smp30(Y/+) mice. Compared with Lepr(db/db)Smp30(Y/+) mice, 24 week old Lepr(db/db)Smp30(Y/-) mice showed: i) increased small dense LDL-cho and decreased HDL-cho levels; ii) fatty liver accompanied by numerous inflammatory cells and increased oxidative stress; iii) decreased mRNA expression of genes involved in fatty acid oxidation (PPARα) and lipoprotein uptake (LDLR and VLDLR) but increased CD36 levels; and iv) increased endoplasmic reticulum stress.
Conclusion: Our data strongly suggest that SMP30 is closely associated with NAFLD pathogenesis, and might be a possible therapeutic target for NAFLD.
Conflict of interest statement
Competing Interests: Vitamin C powder was kindly provided by DSM Nutrition Japan. This does not alter the authors' adherence to all the PLOS ONE policies on sharing data and materials.
Figures
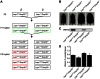
Figure 1. Establishment of Leprdb/dbSmp30Y/− mice.
(A) Generation of Leprdb/dbSmp30Y/− mice. F1 hybrid mice (in green boxes) were crossed to produce the experimental Leprdb/+Smp30Y/+, Leprdb/+Smp30Y/−, Leprdb/dbSmp30Y/+ and Leprdb/dbSmp30Y/− mice (in red boxes). (B) Appearance of Leprdb/dbSmp30Y/− mice at 24 weeks of age. (C) Western blot analysis of SMP30 protein levels in livers from each experimental group at 24 weeks of age. (D) Vitamin C concentration in livers from each experimental group of mice at 24 weeks of age. Values are given as means ± SEM of five animals.
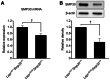
Figure 2. Decrease in hepatic SMP30 levels in Leprdb/db mice.
(A) SMP30 mRNA and (B) protein levels in livers from Leprdb/+Smp30Y/+ and Leprdb/dbSmp30Y/+ mice. Values are given as means ± SEM of five animals. † P<0.01.
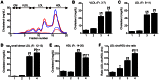
Figure 3. Increase in small dense LDL-cho and decrease in HDL-cho in plasma from Leprdb/dbSmp30Y/− mice.
(A) HPLC lipoprotein profile with cholesterol reagent and (B–F) cholesterol content in four subfractions according particle size, VLDL; Fr. 3–7, LDL; Fr. 8–11, small dense LDL; Fr. 12–13, HDL; Fr. 14–20 and LDL/HDL ratio in the four experimental groups. Blue dashed line and lane 1, Leprdb/+Smp30Y/+ mice; Red dashed line and lane 2, Leprdb/+Smp30Y/− mice; Blue line and lane 3, Leprdb/dbSmp30Y/+ mice; Red line and lane 4; _Leprdb/dbSmp30Y/−_mice. Values are given as means ± SEM of five animals. † P<0.01 and § P<0.001 versus Leprdb/+Smp30Y/+, ¶ P<0.001 versus Leprdb/+Smp30Y/−, ** P<0.05 and †† P<0.001 versus Leprdb/dbSmp30Y/+.
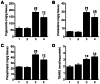
Figure 4. Increase in TBARS in livers from Leprdb/dbSmp30Y/− mice.
(A) Triglyceride, (B) Cholesterol, (C) Phospholipid and (D) TBARS content in livers from the four experimental groups. Lane 1, Leprdb/+Smp30Y/+ mice. Lane 2, Leprdb/+Smp30Y/− mice. Lane 3, Leprdb/dbSmp30Y/+ mice. Lane 4, Leprdb/dbSmp30Y/− mice. † P<0.01 and § P<0.001 versus Leprdb/+Smp30Y/+, ¶ P<0.001 versus Leprdb/+Smp30Y/−, ** P<0.05 and †† P<0.001 versus Leprdb/dbSmp30Y/+. Values are given as means ± SEM of five animals.
Figure 5. Increased steatosis, inflammation and oxidative stress in liver sections from Leprdb/dbSmp30Y/− mice.
Representative images of (A) hematoxylin/eosin staining and (B) 4-HNE immunostaining in liver sections from Leprdb/+Smp30Y/+, Leprdb/+Smp30Y/−, Leprdb/dbSmp30Y/+ and Leprdb/dbSmp30Y/− mice. Scale bar is 100 µm. * P<0.05, † P<0.01 and § P<0.001 versus Leprdb/+Smp30Y/+, # P<0.05, ‡ P<0.01 and ¶ P<0.001 versus Leprdb/+Smp30Y/−, ** P<0.05 versus Leprdb/dbSmp30Y/+. Values are given as mean ± SEM of five animals.
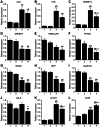
Figure 6. Altered hepatic expression of lipid/lipoprotein metabolism-related genes in Leprdb/dbSmp30Y/− mice.
Gene expression levels of (A) ACC, (B) FAS, (C) SREBP1c, (D) SREBP2, (E) HMGCoAR, (F) _PPAR_α, (G) MCAD, (H) MTP, (I) ApoB100, (J) LDLR (K) VLDLR, and (L) CD36 in the livers. Lane 1: Leprdb/+Smp30Y/+, lane 2: Leprdb/+Smp30Y/−, lane 3: Leprdb/dbSmp30Y/+ and lane 4: Leprdb/dbSmp30 Y/− mice. mRNA for each gene was measured using real time RT-PCR and normalized to β-actin. The values from Leprdb/+ Smp30Y/+ mice were assigned a relative value of 1.0. Values are given as means ± SEM of five animals. * P<0.05, † P<0.01 and § P<0.001 versus Leprdb/+Smp30Y/+, # P<0.05, ‡ P<0.01 and ¶ P<0.001 versus Leprdb/+Smp30Y/−, ** P<0.05 versus Leprdb/dbSmp30Y/+.
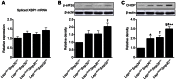
Figure 7. Increase in endoplasmic reticulum (ER) stress in livers from Leprdb/dbSmp30Y/− mice.
(A) Spliced XBP1 mRNA expression, (B) Phosphorylated eIF2α protein and (C) CHOP protein levels in livers from the four experimental groups. Values are given as means ± SEM of five animals. * P<0.05, † P<0.01 and § P<0.001 versus Leprdb/+Smp30Y/+, # P<0.05 versus Leprdb/+Smp30Y/−, ** P<0.05 versus Leprdb/dbSmp30Y/+.
Similar articles
- Adiponectin homolog novel osmotin protects obesity/diabetes-induced NAFLD by upregulating AdipoRs/PPARα signaling in ob/ob and db/db transgenic mouse models.
Ahmad A, Ali T, Kim MW, Khan A, Jo MH, Rehman SU, Khan MS, Abid NB, Khan M, Ullah R, Jo MG, Kim MO. Ahmad A, et al. Metabolism. 2019 Jan;90:31-43. doi: 10.1016/j.metabol.2018.10.004. Epub 2018 Oct 25. Metabolism. 2019. PMID: 30473057 - Cellular cholesterol accumulation modulates high fat high sucrose (HFHS) diet-induced ER stress and hepatic inflammasome activation in the development of non-alcoholic steatohepatitis.
Bashiri A, Nesan D, Tavallaee G, Sue-Chue-Lam I, Chien K, Maguire GF, Naples M, Zhang J, Magomedova L, Adeli K, Cummins CL, Ng DS. Bashiri A, et al. Biochim Biophys Acta. 2016 Jul;1861(7):594-605. doi: 10.1016/j.bbalip.2016.04.005. Epub 2016 Apr 14. Biochim Biophys Acta. 2016. PMID: 27090939 - Involvement of senescence marker protein-30 in glucose metabolism disorder and non-alcoholic fatty liver disease.
Kondo Y, Ishigami A. Kondo Y, et al. Geriatr Gerontol Int. 2016 Mar;16 Suppl 1:4-16. doi: 10.1111/ggi.12722. Geriatr Gerontol Int. 2016. PMID: 27018279 Review. - Involvement of regucalcin in lipid metabolism and diabetes.
Yamaguchi M, Murata T. Yamaguchi M, et al. Metabolism. 2013 Aug;62(8):1045-51. doi: 10.1016/j.metabol.2013.01.023. Epub 2013 Feb 28. Metabolism. 2013. PMID: 23453039 Review.
Cited by
- Underlying Mechanisms and Treatment of Cellular Senescence-Induced Biological Barrier Interruption and Related Diseases.
Sun R, Feng J, Wang J. Sun R, et al. Aging Dis. 2024 Apr 1;15(2):612-639. doi: 10.14336/AD.2023.0621. Aging Dis. 2024. PMID: 37450933 Free PMC article. Review. - The Role of Oxidative Stress and Cellular Senescence in the Pathogenesis of Metabolic Associated Fatty Liver Disease and Related Hepatocellular Carcinoma.
Anastasopoulos NA, Charchanti AV, Barbouti A, Mastoridou EM, Goussia AC, Karampa AD, Christodoulou D, Glantzounis GK. Anastasopoulos NA, et al. Antioxidants (Basel). 2023 Jun 14;12(6):1269. doi: 10.3390/antiox12061269. Antioxidants (Basel). 2023. PMID: 37371999 Free PMC article. Review. - Ascorbate Is a Primary Antioxidant in Mammals.
Fujii J, Osaki T, Bo T. Fujii J, et al. Molecules. 2022 Sep 21;27(19):6187. doi: 10.3390/molecules27196187. Molecules. 2022. PMID: 36234722 Free PMC article. Review. - Modulation of Oxidative Stress-Induced Senescence during Non-Alcoholic Fatty Liver Disease.
Pedroza-Diaz J, Arroyave-Ospina JC, Serna Salas S, Moshage H. Pedroza-Diaz J, et al. Antioxidants (Basel). 2022 May 16;11(5):975. doi: 10.3390/antiox11050975. Antioxidants (Basel). 2022. PMID: 35624839 Free PMC article. Review. - Phosphate and Cellular Senescence.
Hu MC, Moe OW. Hu MC, et al. Adv Exp Med Biol. 2022;1362:55-72. doi: 10.1007/978-3-030-91623-7_7. Adv Exp Med Biol. 2022. PMID: 35288873 Free PMC article.
References
- Ong JP, Younossi ZM (2007) Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis 11: 1–16, vii. - PubMed
- Charlton M (2004) Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol 2: 1048–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This study is supported by the Japan Society for the Promotion of Science (http://www.jsps.go.jp/english/e-grants/index.html) KAKENHI Grant Number 24380073 (A. Ishigami), 23590441 (N. Maruyama), 23790122 (Y. Kondo) and 23591317 (G. Hasegawa). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous