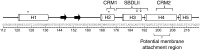Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions - PubMed (original) (raw)
Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions
Jose Luis Ortega-Roldan et al. J Biol Chem. 2013.
Abstract
The sigma-1 receptor (S1R) is a ligand-regulated membrane protein chaperone involved in the ER stress response. S1R activity is implicated in diseases of the central nervous system including amnesia, schizophrenia, depression, Alzheimer disease, and addiction. S1R has been shown previously to regulate the Hsp70 binding immunoglobulin protein (BiP) and the inositol triphosphate receptor calcium channel through a C-terminal domain. We have developed methods for bacterial expression and reconstitution of the chaperone domain of human S1R into detergent micelles that enable its study by solution NMR spectroscopy. The chaperone domain is found to contain a helix at the N terminus followed by a largely dynamic region and a structured, helical C-terminal region that encompasses a membrane associated domain containing four helices. The helical region at residues ∼198-206 is strongly amphipathic and proposed to anchor the chaperone domain to micelles and membranes. Three of the helices in the C-terminal region closely correspond to previously identified cholesterol and drug recognition sites. In addition, it is shown that the chaperone domain interacts with full-length BiP or the isolated nucleotide binding domain of BiP, but not the substrate binding domain, suggesting that the nucleotide binding domain is sufficient for S1R interactions.
Keywords: BiP; Endoplasmic Reticulum Stress; Membrane Proteins; NMR; Sigma-1 Receptor; Signaling; Structural Biology.
Figures
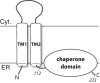
FIGURE 1.
Full-length S1R topology schematic showing two predicted transmembrane helices (TM1 and TM2) and the membrane-associated domain. The human S1R(cd) construct studied here contains residues 112–223 that include a predicted disordered region (residues ∼134–167 (40)) and a predicted membrane-associated domain (residues ∼176–204 (47)). The N and C termini and the approximate positions of residues 112 and 223 (the C-terminal residue) are indicated. The membrane topology is based on Refs. and . TM1, transmembrane domain 1; TM2, transmembrane domain 2; Cyt., cytosol.
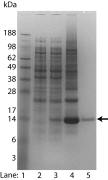
FIGURE 2.
SDS-PAGE stained with Coomassie Blue showing bacterial production of triple-labeled (2H, 13C, and 15N) S1R(cd) using the single protein production system. The S1R(cd) (theoretical mass of 14.7 kDa) band is indicated by an arrow. Lane 1, molecular weight standard; lane 2, whole cell lysate before induction; lane 3, whole cell lysate after induction in minimal medium for 16 h at 15 °C; lane 4, precipitated protein after elution from nickel column; and lane 5, HPLC-purified protein.
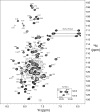
FIGURE 3.
1H,15N HSQC spectrum (600 MHz, 1H) of S1R(cd) in 5 mm DPC at 37 °C. Backbone amide resonance assignments are indicated. The positions of the cross-peaks corresponding to residues Arg-114 and Gly-118, which are weak, are indicated with dashed line circles. The five cross-peak pairs expected from asparagine and glutamine H2N(ϵ2) groups of the S1R(cd) construct are indicated with horizontal lines. Inset, the four tryptophan HN(ϵ1) cross-peaks.
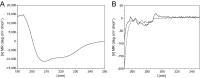
FIGURE 4.
Circular dichroism spectra of S1R(cd) in 5 mm DPC and 20 mm HEPES, pH 6.5, plotted as the mean residue molar ellipticity ([θ] MR). A, far UV spectrum of S1R(cd) (17.5 μ
m
) at 37 °C. B, near UV spectra of S1R(cd) (240 μ
m
) at 37 °C (solid line) and 95 °C (dashed line).
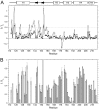
FIGURE 5.
A, summary of NOE cross-peaks to backbone amide protons. Amide proton to amide proton NOEs (dNN(i+1)), and amide proton to α-proton NOEs (d_α_N(i+1), d_α_N(i+2), and d_α_N(i+3)), as well as cross-peaks at the position of the water proton (dN H_2_O), were measured in a three-dimensional 15N-edited NOESY with 180-ms mixing time at 600 MHz (1H) on an 15N-labeled S1R(cd) in 5 m
m
DPC. Amide proton to DPC methyl proton NOEs (dN DPC (_CH_3)) were obtained from a three-dimensional 15N-edited NOESY with 90 ms of mixing time at 950 MHz (1H) on an 15N- and 2H-labeled (∼70%) S1R(cd) in 30 m
m
DPC. The x axis (arbitrary units) is the cross-peak intensity normalized to that of the diagonal. No attempt was made to deconvolute overlapped cross-peaks. B, the chemical shift indices (CSI) of 1Hα, 13Cα, 13C′, and 13Cβ are shown in the top four panels. In panels five and six are shown the secondary structure and random coil index derived from chemical shift analysis using TALOS+ (49) and the method of Berjanskii et al. (50), respectively. For the TALOS+ plot, values above or below the line indicated that the calculated φ/ψ values correspond to helical (H) or extended conformation (E), respectively. Shown schematically at the top are the S1R(cd) residues determined to be helical (cylinders) or extended (arrow) (see main text for full description of secondary structure assignment).
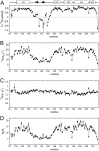
FIGURE 6.
Relaxation properties of S1R(cd). A, 1H-15N heteronuclear NOEs (het-NOE); B, 15N transverse relaxation rates (15N R2); C, 15N longitudinal relaxation rates (15N R1); and D, ratio of 15N R2 and R1, as a function of residue number for S1R(cd) at 600 MHz (1H).
FIGURE 7.
Summary of water interactions with S1R(cd). A, backbone amide proton exchange of S1R(cd) with water measured using CLEANEX experiments (25). Water proton exchange is shown as the ratio of the peak volume in CLEANEX experiments using 10-ms (filled circles) or 30-ms (open squares) mixing times to the peak volume in a fast HSQC (600 MHz 1H). Measurement error has been estimated from the spectral noise. B, resonance broadening after addition of the water-soluble paramagnetic agent Mn2+EDDA2−. A decreased ratio of the peak intensity after (I) and before (_I_o) addition of Mn2+EDDA2− indicates increased water accessibility. Measurement error has been estimated from the spectral noise. Data for resonances that exhibit high water exchange rates as indicated by CLEANEX ratios of I/_I_o > 0.4 (dashed line in A) were excluded from the analysis due to potential artifacts in cross-peak intensities due to changes in relaxation properties.
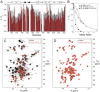
FIGURE 8.
S1R(cd) interactions with BiP. A, backbone amide cross-peak intensities are plotted as a function of residue number after addition of 0.5 (black bars) and 1.0 (red bars) molar equivalents of full-length BiP (no calcium). The intensities are normalized to the cross-peak intensities in the absence of BiP. B, the normalized average backbone amide cross-peak intensity of S1R(cd) as a function of the molar ratio of BiP NBD (no calcium) or full-length BiP to S1R(cd) in the absence or presence of 2.5 m
m
calcium chloride. The intensity average is for only the helical regions. C, spectral overlays of 15N-labeled S1R(cd) with (red) or without (black) full-length BiP (1:1) in the presence of 2.5 m
m
calcium. D, spectral overlays of 15N-labeled S1R(cd) with (red) and without (black) addition of the BiP SBD (1:1) in the presence of 2.5 m
m
calcium.
FIGURE 9.
The helical (cylinders) and extended (arrow) residues of S1R(cd) determined from a combination of chemical shifts, amide-water proton exchange, 15N relaxation rates, and NOEs. Residues and regions previously implicated in cholesterol binding are indicated: cholesterol binding motifs (CRM1 and CRM2), cocaine binding (SBDLII; residues Asp-188 and Val-190 are indicated by ↓), and haloperidol binding (residues Asp-126 and Glu-172 are indicated by an asterisk). Residues 198–206, which are proposed to have the strongest interactions with the ER membrane, are indicated. The amino acid sequence and corresponding residue numbers are shown at the bottom.
Similar articles
- Solution NMR studies reveal the location of the second transmembrane domain of the human sigma-1 receptor.
Ortega-Roldan JL, Ossa F, Amin NT, Schnell JR. Ortega-Roldan JL, et al. FEBS Lett. 2015 Feb 27;589(5):659-65. doi: 10.1016/j.febslet.2015.01.033. Epub 2015 Jan 31. FEBS Lett. 2015. PMID: 25647032 Free PMC article. - Biochemical Pharmacology of the Sigma-1 Receptor.
Chu UB, Ruoho AE. Chu UB, et al. Mol Pharmacol. 2016 Jan;89(1):142-53. doi: 10.1124/mol.115.101170. Epub 2015 Nov 11. Mol Pharmacol. 2016. PMID: 26560551 Review. - Pridopidine reduces mutant huntingtin-induced endoplasmic reticulum stress by modulation of the Sigma-1 receptor.
Shenkman M, Geva M, Gershoni-Emek N, Hayden MR, Lederkremer GZ. Shenkman M, et al. J Neurochem. 2021 Jul;158(2):467-481. doi: 10.1111/jnc.15366. Epub 2021 Apr 28. J Neurochem. 2021. PMID: 33871049 - The role of sigma 1 receptor in organization of endoplasmic reticulum signaling microdomains.
Zhemkov V, Ditlev JA, Lee WR, Wilson M, Liou J, Rosen MK, Bezprozvanny I. Zhemkov V, et al. Elife. 2021 May 11;10:e65192. doi: 10.7554/eLife.65192. Elife. 2021. PMID: 33973848 Free PMC article. - HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum.
Wang J, Lee J, Liem D, Ping P. Wang J, et al. Gene. 2017 Jun 30;618:14-23. doi: 10.1016/j.gene.2017.03.005. Epub 2017 Mar 7. Gene. 2017. PMID: 28286085 Free PMC article. Review.
Cited by
- Deferred Administration of Afobazole Induces Sigma1R-Dependent Restoration of Striatal Dopamine Content in a Mouse Model of Parkinson's Disease.
Kadnikov IA, Verbovaya ER, Voronkov DN, Voronin MV, Seredenin SB. Kadnikov IA, et al. Int J Mol Sci. 2020 Oct 15;21(20):7620. doi: 10.3390/ijms21207620. Int J Mol Sci. 2020. PMID: 33076300 Free PMC article. - Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases.
Ryskamp DA, Korban S, Zhemkov V, Kraskovskaya N, Bezprozvanny I. Ryskamp DA, et al. Front Neurosci. 2019 Aug 28;13:862. doi: 10.3389/fnins.2019.00862. eCollection 2019. Front Neurosci. 2019. PMID: 31551669 Free PMC article. Review. - Role of Sigma-1 Receptor in Calcium Modulation: Possible Involvement in Cancer.
Pontisso I, Combettes L. Pontisso I, et al. Genes (Basel). 2021 Jan 22;12(2):139. doi: 10.3390/genes12020139. Genes (Basel). 2021. PMID: 33499031 Free PMC article. Review. - Sigma 1 receptor regulates the oxidative stress response in primary retinal Müller glial cells via NRF2 signaling and system xc(-), the Na(+)-independent glutamate-cystine exchanger.
Wang J, Shanmugam A, Markand S, Zorrilla E, Ganapathy V, Smith SB. Wang J, et al. Free Radic Biol Med. 2015 Sep;86:25-36. doi: 10.1016/j.freeradbiomed.2015.04.009. Epub 2015 Apr 25. Free Radic Biol Med. 2015. PMID: 25920363 Free PMC article. - Sphingoid Bases Regulate the Sigma-1 Receptor-Sphingosine and N,N'-Dimethylsphingosine Are Endogenous Agonists.
Li J, Satyshur KA, Guo LW, Ruoho AE. Li J, et al. Int J Mol Sci. 2023 Feb 4;24(4):3103. doi: 10.3390/ijms24043103. Int J Mol Sci. 2023. PMID: 36834510 Free PMC article.
References
- Hayashi T., Su T. P. (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131, 596–610 - PubMed
- Walter L., Hajnóczky G. (2005) Mitochondria and endoplasmic reticulum: the lethal interorganelle cross-talk. J. Bioenerg. Biomembr. 37, 191–206 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources