Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes - PubMed (original) (raw)
Randomized Controlled Trial
. 2013 Oct;62(10):3532-41.
doi: 10.2337/db13-0581. Epub 2013 Jun 12.
Affiliations
- PMID: 23761104
- PMCID: PMC3781477
- DOI: 10.2337/db13-0581
Randomized Controlled Trial
Disordered control of intestinal sweet taste receptor expression and glucose absorption in type 2 diabetes
Richard L Young et al. Diabetes. 2013 Oct.
Abstract
We previously established that the intestinal sweet taste receptors (STRs), T1R2 and T1R3, were expressed in distinct epithelial cells in the human proximal intestine and that their transcript levels varied with glycemic status in patients with type 2 diabetes. Here we determined whether STR expression was 1) acutely regulated by changes in luminal and systemic glucose levels, 2) disordered in type 2 diabetes, and 3) linked to glucose absorption. Fourteen healthy subjects and 13 patients with type 2 diabetes were studied twice, at euglycemia (5.2 ± 0.2 mmol/L) or hyperglycemia (12.3 ± 0.2 mmol/L). Endoscopic biopsy specimens were collected from the duodenum at baseline and after a 30-min intraduodenal glucose infusion of 30 g/150 mL water plus 3 g 3-O-methylglucose (3-OMG). STR transcripts were quantified by RT-PCR, and plasma was assayed for 3-OMG concentration. Intestinal STR transcript levels at baseline were unaffected by acute variations in glycemia in healthy subjects and in type 2 diabetic patients. T1R2 transcript levels increased after luminal glucose infusion in both groups during euglycemia (+5.8 × 10(4) and +5.8 × 10(4) copies, respectively) but decreased in healthy subjects during hyperglycemia (-1.4 × 10(4) copies). T1R2 levels increased significantly in type 2 diabetic patients under the same conditions (+6.9 × 10(5) copies). Plasma 3-OMG concentrations were significantly higher in type 2 diabetic patients than in healthy control subjects during acute hyperglycemia. Intestinal T1R2 expression is reciprocally regulated by luminal glucose in health according to glycemic status but is disordered in type 2 diabetes during acute hyperglycemia. This defect may enhance glucose absorption in type 2 diabetic patients and exacerbate postprandial hyperglycemia.
Figures
FIG. 1.
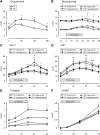
Effects of oral glucose or intraduodenal (ID) glucose infusion on blood glucose levels and plasma levels of hormones and the glucose absorption marker 3-OMG in healthy control (HC) subjects and type 2 diabetic (T2D) patients during euglycemia or hyperglycemia. (A) Blood glucose levels after a glucose drink in HC subjects and T2D patients. *P < 0.05, #P < 0.01, δ_P_ < 0.001 T2D compared with HC. (B) Blood glucose levels after ID glucose infusion during glycemic clamp. δ_P_ < 0.001 HC euglycemic compared with hyperglycemic groups and T2D euglycemic compared with T2D hyperglycemic; *P < 0.05 T2D euglycemic compared with HC euglycemic; #P < 0.05 T2D hyperglycemic compared with HC hyperglycemic. (C) Plasma GLP-1. *P < 0.05 T2D groups compared with HC euglycemic; #P < 0.01 T2D groups compared with HC hyperglycemic. (D) Plasma GIP. *P < 0.05 T2D groups compared with HC euglycemic; **P < 0.05 T2D groups compared with HC hyperglycemic; ***P < 0.05 T2D euglycemic compared with HC groups. (E) C-peptide. δ_P_ < 0.001 HC hyperglycemic compared with euglycemic groups; *P < 0.05 T2D hyperglycemic compared with other groups. (F) 3-OMG. δ_P_ < 0.001 T2D hyperglycemic compared with other groups. Data are mean ± SEM; significance represents treatment × time interactions.
FIG. 2.
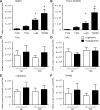
Absolute transcript levels of STR in the duodenum of healthy subjects and type 2 diabetic patients at stable euglycemia and hyperglycemia. Absolute expression (copy number) of STR transcripts at baseline in the duodenum of healthy subjects (A) or patients with type 2 diabetes (B). (A) TRPM5 levels were 15-fold higher, α-gustducin 9-fold higher, and T1R3 3-fold higher than T1R2 levels in healthy subjects. (B) TRPM5 levels were 29-fold higher, α-gustducin 11-fold higher, and T1R3 5-fold higher than T1R2 levels in patients with type 2 diabetes. *P < 0.05 and #P < 0.01 compared with T1R2. Duodenal levels of T1R2 (C), T1R3 (D), α-gustducin (E), and TRPM5 (F) transcript in healthy control (HC) subjects and type 2 diabetic (T2D) patients at stable euglycemia or hyperglycemia. No significant differences in transcript levels were detected at stable baseline. Data are mean ± SEM. α-GD, α-gustducin
FIG. 3.
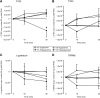
Effects of intraduodenal glucose infusion on sweet taste molecule transcript levels in healthy control (HC) subjects and type 2 diabetic (T2D) patients during euglycemia or hyperglycemia. (A) Change in absolute expression of T1R2 in human duodenum during intraduodenal glucose infusion under euglycemic or hyperglycemic clamp. #P < 0.01 HC hyperglycemic compared with all other groups. T1R3 (B), α-gustducin (C), and TRPM5 (D). Data are mean ± SEM.
FIG. 4.
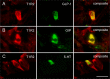
Subsets of L cells, K cells, and EC cells express STR in healthy human duodenum. (A) Immunolabeling for GLP-1 was present in 19 ± 11% of T1R2-labeled duodenal cells in healthy control subjects at euglycemia, whereas 13 ± 8% of L cells coexpressed T1R2. (B) GIP was present in 15 ± 10% of T1R2-labeled cells in healthy control subjects at euglycemia, whereas 12 ± 8% of K cells coexpressed T1R2. (C) In a similar manner, separate populations of T1R2-labeled cells coexpressed 5-HT (31 ± 6%), whereas 5 ± 1% of EC cells coexpressed T1R2. (A_–_C) Scale bar = 20 µm.
Comment in
- How sweet it is: intestinal sweet taste receptors in type 2 diabetes.
Greenfield JR, Chisholm DJ. Greenfield JR, et al. Diabetes. 2013 Oct;62(10):3336-7. doi: 10.2337/db13-1018. Diabetes. 2013. PMID: 24065793 Free PMC article. No abstract available.
Similar articles
- Effects of intraduodenal hydroxycitrate on glucose absorption, incretin release, and glycemia in response to intraduodenal glucose infusion in health and type 2 diabetes: A randomised controlled trial.
Thazhath SS, Wu T, Bound MJ, Checklin HL, Standfield S, Jones KL, Horowitz M, Rayner CK. Thazhath SS, et al. Nutrition. 2016 May;32(5):553-9. doi: 10.1016/j.nut.2015.11.004. Epub 2015 Dec 7. Nutrition. 2016. PMID: 26792024 Clinical Trial. - How sweet it is: intestinal sweet taste receptors in type 2 diabetes.
Greenfield JR, Chisholm DJ. Greenfield JR, et al. Diabetes. 2013 Oct;62(10):3336-7. doi: 10.2337/db13-1018. Diabetes. 2013. PMID: 24065793 Free PMC article. No abstract available. - Expression of sweet taste receptor and gut hormone secretion in modelled type 2 diabetes.
Feng R, Qian C, Liu Q, Jin Y, Liu L, Li S, Liao Y, Zhou H, Liu W, Rayner CK, Ma J. Feng R, et al. Gen Comp Endocrinol. 2017 Oct 1;252:142-149. doi: 10.1016/j.ygcen.2017.08.008. Epub 2017 Aug 4. Gen Comp Endocrinol. 2017. PMID: 28782537 - Postprandial peaks as a risk factor for cardiovascular disease: epidemiological perspectives.
Bonora E. Bonora E. Int J Clin Pract Suppl. 2002 Jul;(129):5-11. Int J Clin Pract Suppl. 2002. PMID: 12166607 Review. - Postprandial glucose regulation: new data and new implications.
Leiter LA, Ceriello A, Davidson JA, Hanefeld M, Monnier L, Owens DR, Tajima N, Tuomilehto J; International Prandial Glucose Regulation Study Group. Leiter LA, et al. Clin Ther. 2005;27 Suppl B:S42-56. doi: 10.1016/j.clinthera.2005.11.020. Clin Ther. 2005. PMID: 16519037 Review.
Cited by
- Lipid stimulation of fatty acid sensors in the human duodenum: relationship with gastrointestinal hormones, BMI and diet.
Cvijanovic N, Isaacs NJ, Rayner CK, Feinle-Bisset C, Young RL, Little TJ. Cvijanovic N, et al. Int J Obes (Lond). 2017 Feb;41(2):233-239. doi: 10.1038/ijo.2016.199. Epub 2016 Nov 4. Int J Obes (Lond). 2017. PMID: 27811952 Clinical Trial. - The Gastrointestinal Tract as Prime Site for Cardiometabolic Protection by Dietary Polyphenols.
Villa-Rodriguez JA, Ifie I, Gonzalez-Aguilar GA, Roopchand DE. Villa-Rodriguez JA, et al. Adv Nutr. 2019 Nov 1;10(6):999-1011. doi: 10.1093/advances/nmz038. Adv Nutr. 2019. PMID: 31144710 Free PMC article. Review. - Gut chemosensing mechanisms.
Psichas A, Reimann F, Gribble FM. Psichas A, et al. J Clin Invest. 2015 Mar 2;125(3):908-17. doi: 10.1172/JCI76309. Epub 2015 Feb 9. J Clin Invest. 2015. PMID: 25664852 Free PMC article. Review. - Manipulation of Post-Prandial Hyperglycaemia in Type 2 Diabetes: An Update for Practitioners.
Shibib L, Al-Qaisi M, Guess N, Miras AD, Greenwald SE, Pelling M, Ahmed A. Shibib L, et al. Diabetes Metab Syndr Obes. 2024 Aug 23;17:3111-3130. doi: 10.2147/DMSO.S458894. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39206417 Free PMC article. Review. - The Function of Gastrointestinal Hormones in Obesity-Implications for the Regulation of Energy Intake.
Farhadipour M, Depoortere I. Farhadipour M, et al. Nutrients. 2021 May 27;13(6):1839. doi: 10.3390/nu13061839. Nutrients. 2021. PMID: 34072172 Free PMC article. Review.
References
- Raybould HE, Hölzer H. Dual capsaicin-sensitive afferent pathways mediate inhibition of gastric emptying in rat induced by intestinal carbohydrate. Neurosci Lett 1992;141:236–238 - PubMed
- Horowitz M, Edelbroek MA, Wishart JM, Straathof JW. Relationship between oral glucose tolerance and gastric emptying in normal healthy subjects. Diabetologia 1993;36:857–862 - PubMed
- Pilichiewicz AN, Chaikomin R, Brennan IM, et al. Load-dependent effects of duodenal glucose on glycemia, gastrointestinal hormones, antropyloroduodenal motility, and energy intake in healthy men. Am J Physiol Endocrinol Metab 2007;293:E743–E753 - PubMed
- Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab 2004;287:E199–E206 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical