Increased long chain acyl-Coa synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles - PubMed (original) (raw)
Increased long chain acyl-Coa synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles
Jules A Nchoutmboube et al. PLoS Pathog. 2013.
Abstract
All positive strand (+RNA) viruses of eukaryotes replicate their genomes in association with membranes. The mechanisms of membrane remodeling in infected cells represent attractive targets for designing future therapeutics, but our understanding of this process is very limited. Elements of autophagy and/or the secretory pathway were proposed to be hijacked for building of picornavirus replication organelles. However, even closely related viruses differ significantly in their requirements for components of these pathways. We demonstrate here that infection with diverse picornaviruses rapidly activates import of long chain fatty acids. While in non-infected cells the imported fatty acids are channeled to lipid droplets, in infected cells the synthesis of neutral lipids is shut down and the fatty acids are utilized in highly up-regulated phosphatidylcholine synthesis. Thus the replication organelles are likely built from de novo synthesized membrane material, rather than from the remodeled pre-existing membranes. We show that activation of fatty acid import is linked to the up-regulation of cellular long chain acyl-CoA synthetase activity and identify the long chain acyl-CoA syntheatse3 (Acsl3) as a novel host factor required for polio replication. Poliovirus protein 2A is required to trigger the activation of import of fatty acids independent of its protease activity. Shift in fatty acid import preferences by infected cells results in synthesis of phosphatidylcholines different from those in uninfected cells, arguing that the viral replication organelles possess unique properties compared to the pre-existing membranes. Our data show how poliovirus can change the overall cellular membrane homeostasis by targeting one critical process. They explain earlier observations of increased phospholipid synthesis in infected cells and suggest a simple model of the structural development of the membranous scaffold of replication complexes of picorna-like viruses, that may be relevant for other (+)RNA viruses as well.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
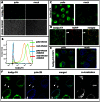
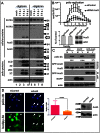
Figure 1. Poliovirus infection induces strong activation of fatty acid import.
HeLa cells were infected with poliovirus at 50 PFU/cell, and at 4 h p. i. Bodipy 500/510 C4–C9 (bodipy-FA) was added for 30 min. A. low magnification view of infected vs mock-infected cells. B. FACS analysis of the fluorescence of infected (mock-infected) cells after 30 min pulse label with bodipy-FA at 4 h p. I, or control samples incubated without bodipy-FA. C. Higher magnification image showing that bodipy-FA probe is redistributed preferentially into lipid droplets in mock-infected cells and into membranes in infected cells. D. A confocal image of HeLa cells expressing pmCherry-ADRP protein (a marker for lipid droplets) and labeled with bodipy-FA for 30 min. Arrow marks a lipid droplet that did not accumulate the newly-synthesized lipids during the labeling period and also indicate lack of bodipy fluorescence leakage into the red channel. E. A confocal image of infected (mock-infected) HeLa cells labeled at 4 h p. i. with bodipy-FA and Hoechst-33342 (cell permeable DNA stain) for 30 min showing localization of bodipy-FA staining. F. A confocal image of polio-infected HeLa cells incubated for 30 min with bodipy-FA at 4 h.p.i. and processed for staining for a viral membrane-targeted protein 2B showing co-localization of the viral antigen and imported FA. Colocalization panel shows colocalized green and blue pixels identified with ImageJ software.
Figure 2. In infected cells synthesis of neutral lipids is shut down and imported fatty acids are directed to strongly stimulated synthesis of phosphatidylcholine.
HeLa cells were infected (V) or mock-infected (M) with poliovirus at 50 PFU/cell and incubated in medium with or without serum as indicated. Bodipy-FA was added for 30 min at 4 h p. i.; after that total lipids were extracted and resolved by thin layer chromatography. A. Neutral lipids resolved in hexane∶ether∶acetic acid (80∶20∶1) system. Bodipy-FA-lipids represent fluorescent fatty acid-containing lipids synthesized during 30 min labeling period. Total lipids show all neutral lipids stained with bromothymol. B. Polar lipids resolved in chloroform∶ethanol∶water∶triethylamine (30∶35∶7∶35) system. Bodipy-FA-lipids represent fluorescent fatty acid-containing lipids synthesized during 30 min labeling period. Total lipids show all phospholipids stained with Phostain. PC: 2-Oleoyl-1-palmitoyl-sn-glycero-3-phosphocholine (phosphatidylcholine marker).
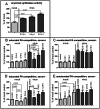
Figure 3. Modulation of long chain acyl-CoA synthetase activity in poliovirus-infected cells.
A. Acyl-CoA synthetase activity is stimulated as early as 2 h p. i. and continues to increase during the time-course of infection. Acyl-CoA synthetase activity in vitro assay was performed with lysates of HeLa cells collected at indicated times post infection, the data are normalized to the activity of the lysate from mock-infected collected at 2 h p. i., p-values are shown. B. and C. Fatty import competition assay performed with the cells incubated in serum- supplemented medium during the whole experiment. HeLa cells were infected (or mock-infected) with poliovirus at 50 PFU/cell; at 4 h.p.i. bodipy-FA was added for 30 min in the presence of 125× molar excess of the indicated long chain fatty acids. No competitor fatty acid was added to control samples. The data are normalized to the signal from the mock-infected control sample; p-values are shown. D. and E. Fatty import competition assay performed with the cells incubated in serum-free medium during the whole experiment. HeLa cells were infected (or mock-infected) with poliovirus at 50 PFU/cell; at 4 h.p.i. bodipy-FA was added for 30 min in the presence of 125× molar excess of the indicated long chain fatty acids. No competitor fatty acid was added to control samples. The data are normalized to the signal from the mock-infected control sample; p-values indicating significant differences are shown.
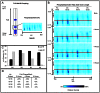
Figure 4. Shift in phosphatidylcholine spectrum following infection demonstrated by TLC-MALDI.
A. Schematic representation of TLC-MALDI, blue spots represent phospholipid migration on TLC plate, hash marks represent stepwise MALDI data capture. B. HeLa cells were infected with poliovirus at 50 PFU/cell and processed for the total lipid extraction at the indicated time points post infection. TLC-MALDI data shown at phosphatidylcholine (PC) migration (Rf) range, fatty acid chain lengths noted, intensity of signal at respective mass to charge ratios (m/z) (blue scale). C. PC diversity (unique m/z signatures) shifts in abundance from higher Rf to lower Rf during time course of infection. D. Percent change of PC subset/PC total ratio compared to mock. Results from a representative experiment are shown.
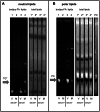
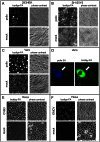
Figure 5. Cleavage and redistribution of long chain acyl-CoA synthetases in infected cells and requirement of functional Acsl3 for polio replication and FA import.
A. HeLa cells infected at 50 PFU/cells were incubated for 2, 4, and 6 hours post infection and collected for Western blot after permeabilization with digitonin for 5 min at room temperature (lanes 5–8); control cells (lanes 1–4) underwent the same treatment but without the detergent. Proteins were detected by multiple western blots of the same membrane after stripping of previous antibodies. Actin is shown as loading control. Results from a representative experiment are shown. Arrows indicate cleavage products detected with anti-FATP3 and Acsl3 antibodies. Arrowhead points to the loss of FATP3 after digitonin treatment from infected cells. B. Acsl3 knock-down severely impairs polio replicon replication (top panel) while showing minimal cytotoxicity (lower panel). siRNA knock-down efficiency of Acsl3 protein is shown. C. Expression of a fusion protein GFP-Acsl3-HA reduces poliovirus replication. HeLa cells were transfected overnight with either empty pUC plasmid, pEGFP-N1 plasmid or pGFP-Acsl3-HA plasmid. Cells were infected (V) with poliovirus at 50 PFU/cell or mock-infected (M) and collected for analysis at 4 h p.i. Polio 2C band intensity is normalized to the EGFP expressing sample. Expression of GFP-Acsl3 protein is detected with either anti-Acsl3 antibodies (second panel) or anti-GFP antibodies (third panel) which also show expression of EGFP (forth panel). Actin is shown as loading control. D. Knock-down of ACSL3 expression reduces activation of FA import upon expression of poliovirus proteins. HeLa cells were transfected with control or ACSL-3-targeting siRNA and 48 h later they were transfected with the plasmid pTM-2A-3D coding for the entire poliovirus non-structural polyprotein fragment P2P3. The next day expression of polio proteins was induced by infection of cells with vaccinia-T7 virus. Bodipy-FA label was added for 30 min at 4 h post vaccinia-T7 infection. Statistical analysis of ∼150 cells from each sample shows bodipy-FA signal normalized to poliovirus antigen 2B fluorescence, p value is shown. Western blot shows ACSL3 knock-down, actin is shown as a loading control.
Figure 6. Activation of fatty acid import requires polio protein 2A.
A. Schematic representation of poliovirus genome and truncated constructs used for expression of polio proteins. B. Only expression of the full P2P3 region activates import of bodipy-FA label (Arrows). HeLa cells were transfected with plasmids coding for the indicated polyprotein fragments under control of T7 promoter (empty vector for the control sample) The next day the cells were infected with vaccinia-T7 virus and labeled with bodipy-FA for 30 min at 4 h p. i. Poliovirus antigen 3A is detected as a marker of expression of viral polyprotein fragments 2A-3D (complete P2–P3), 2B-3D and 2C-3D. C. Protease activity of 2A is dispensable for activation of fatty acid import (arrows). HeLa cells were transfected with plasmids coding for the indicated polyprotein fragments under control of T7 promoter (empty vector for the control sample) The next day the cells were infected with vaccinia-T7 virus and labeled with bodipy-FA for 30 min at 4 h p. i. Poliovirus antigen 2B is detected as a marker of expression of the wt and 2A-mut containing P2–P3 polyprotein. D. Parallel samples to those shown in C were collected and analyzed for 2A protease activity and expression of viral proteins. Processing of eIF-4G (black arrow) is detected only in the sample expressing functional 2A protease (top panel). Accumulation of viral proteins detected by viral antigen 3D is comparable in all samples showing that the lack of 2A protease activity is because of the mutation, not because of the insufficient expression (lower panel).
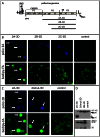
Figure 7. Activation of long chain fatty acid import is a general phenomenon of picornavirus infection.
A–C. 293HEK (human embryonic kidney), SH-S5Y5 (human neuroblastoma) or Vero (green African kidney) cells were infected with poliovirus at 50 PFU/cell and incubated for 4 hours before 30 min label with bodipy-FA. Fluorescent and phase contrast images of infected and mock-infected cells are shown. D. Higher magnification of Vero cells infected with poliovirus and labeled with bodipy-FA like in C, showing that bodipy-FA accumulation is activated only in cells actively expressing viral proteins (arrow). E, F. HeLa cells were infected with either Coxsackie B3 virus or encephalomyocarditis virus at 50 PFU/cell and incubated for 4 hours before 30 min label with bodipy-FA. Fluorescent and phase contrast images of infected and mock-infected cells are shown.
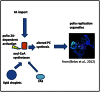
Figure 8. A model of the structural development of picornavirus replication complexes based on activation of cellular acyl-CoA synthetase activity by viral proteins (2A is required, but is not sufficient in case of polio).
Elevated acyl-CoA synthetase activity leads to increased import of fatty acids (FA) form the media and also to utilization of FA form intracellular sources (FAS- fatty acid synthase)resulting in upregulated synthesis of altered species of phosphatidylcholines (PC). PCs with short fatty acid moieties preferentially synthesized in polio-infected cells spontaneously assemble into characteristic tightly packed convoluted tubular membranes structures constituting structural scaffold of poliovirus replication organelles.
Similar articles
- Phospholipid synthesis fueled by lipid droplets drives the structural development of poliovirus replication organelles.
Viktorova EG, Nchoutmboube JA, Ford-Siltz LA, Iverson E, Belov GA. Viktorova EG, et al. PLoS Pathog. 2018 Aug 27;14(8):e1007280. doi: 10.1371/journal.ppat.1007280. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30148882 Free PMC article. - Fluorescent fatty acid analogs as a tool to study development of the picornavirus replication organelles.
Viktorova EG, Ford-Siltz LA, Nchoutmboube J, Belov GA. Viktorova EG, et al. J Virol Methods. 2014 May;200:15-21. doi: 10.1016/j.jviromet.2014.01.020. Epub 2014 Feb 3. J Virol Methods. 2014. PMID: 24503038 - Inhibition of poliovirus-induced cleavage of cellular protein PCBP2 reduces the levels of viral RNA replication.
Chase AJ, Daijogo S, Semler BL. Chase AJ, et al. J Virol. 2014 Mar;88(6):3192-201. doi: 10.1128/JVI.02503-13. Epub 2013 Dec 26. J Virol. 2014. PMID: 24371074 Free PMC article. - A new concept of cellular uptake and intracellular trafficking of long-chain fatty acids.
Stremmel W, Pohl L, Ring A, Herrmann T. Stremmel W, et al. Lipids. 2001 Sep;36(9):981-9. doi: 10.1007/s11745-001-0809-2. Lipids. 2001. PMID: 11724471 Review. - Involvement of cellular membrane traffic proteins in poliovirus replication.
Belov GA, Ehrenfeld E. Belov GA, et al. Cell Cycle. 2007 Jan 1;6(1):36-8. doi: 10.4161/cc.6.1.3683. Epub 2007 Jan 11. Cell Cycle. 2007. PMID: 17245115 Review.
Cited by
- The class III phosphatidylinositol 3-kinase VPS34 supports EV71 replication by promoting viral replication organelle formation.
Wu B, Fan T, Chen X, He Y, Wang H. Wu B, et al. J Virol. 2024 Oct 22;98(10):e0069524. doi: 10.1128/jvi.00695-24. Epub 2024 Sep 10. J Virol. 2024. PMID: 39254312 - Identification of the proteolytic signature in CVB3-infected cells.
Vlok M, Solis N, Sadasivan J, Mohamud Y, Warsaba R, Kizhakkedathu J, Luo H, Overall CM, Jan E. Vlok M, et al. J Virol. 2024 Jul 23;98(7):e0049824. doi: 10.1128/jvi.00498-24. Epub 2024 Jul 2. J Virol. 2024. PMID: 38953667 Free PMC article. - Picornavirus security proteins promote the release of extracellular vesicle enclosed viruses via the modulation of host kinases.
Defourny KAY, Pei X, van Kuppeveld FJM, Nolte-T Hoen ENM. Defourny KAY, et al. PLoS Pathog. 2024 Apr 25;20(4):e1012133. doi: 10.1371/journal.ppat.1012133. eCollection 2024 Apr. PLoS Pathog. 2024. PMID: 38662794 Free PMC article. - Leishmania infection upregulates and engages host macrophage Argonaute 1, and system-wide proteomics reveals Argonaute 1-dependent host response.
Moradimotlagh A, Chen S, Koohbor S, Moon KM, Foster LJ, Reiner N, Nandan D. Moradimotlagh A, et al. Front Immunol. 2023 Nov 30;14:1287539. doi: 10.3389/fimmu.2023.1287539. eCollection 2023. Front Immunol. 2023. PMID: 38098491 Free PMC article. - Organelles are miscommunicating: Membrane contact sites getting hijacked by pathogens.
Paul P, Tiwari B. Paul P, et al. Virulence. 2023 Dec;14(1):2265095. doi: 10.1080/21505594.2023.2265095. Epub 2023 Oct 20. Virulence. 2023. PMID: 37862470 Free PMC article. Review.
References
- Ehrenfeld E, Domingo E, Roos RP, American Society for Microbiology. (2010) The picornaviruses. Washington, DC: ASM Press. xiv, 493 p. p.
Publication types
MeSH terms
Substances
Grants and funding
- NS062043/NS/NINDS NIH HHS/United States
- NS037355/NS/NINDS NIH HHS/United States
- R01 NS062043/NS/NINDS NIH HHS/United States
- T32 AI007540/AI/NIAID NIH HHS/United States
- HD024061/HD/NICHD NIH HHS/United States
- R01 NS037355/NS/NINDS NIH HHS/United States
- P30 HD024061/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous