Two RNA-binding motifs in eIF3 direct HCV IRES-dependent translation - PubMed (original) (raw)
Two RNA-binding motifs in eIF3 direct HCV IRES-dependent translation
Chaomin Sun et al. Nucleic Acids Res. 2013 Aug.
Abstract
The initiation of protein synthesis plays an essential regulatory role in human biology. At the center of the initiation pathway, the 13-subunit eukaryotic translation initiation factor 3 (eIF3) controls access of other initiation factors and mRNA to the ribosome by unknown mechanisms. Using electron microscopy (EM), bioinformatics and biochemical experiments, we identify two highly conserved RNA-binding motifs in eIF3 that direct translation initiation from the hepatitis C virus internal ribosome entry site (HCV IRES) RNA. Mutations in the RNA-binding motif of subunit eIF3a weaken eIF3 binding to the HCV IRES and the 40S ribosomal subunit, thereby suppressing eIF2-dependent recognition of the start codon. Mutations in the eIF3c RNA-binding motif also reduce 40S ribosomal subunit binding to eIF3, and inhibit eIF5B-dependent steps downstream of start codon recognition. These results provide the first connection between the structure of the central translation initiation factor eIF3 and recognition of the HCV genomic RNA start codon, molecular interactions that likely extend to the human transcriptome.
Figures
Figure 1.
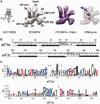
Cryo-EM reconstructions of eIF3 8-mer in its apo and HCV IRES IIIabc-bound form, and sequence analyses of eIF3 subunits a* and c*. (A) 3D reconstruction of the eIF3 8-mer (in gray) compared with the 8-mer/IIIabc complex (in purple) and difference map (solid purple positive density at a threshold of 5 sigma on a mesh representation of the apo-8-mer). The secondary structure of the HCV IRES and IIIabc region are shown to the left. Anthropomorphic features of eIF3, as indicated in (14), are also indicated. (B) Sequence analyses of the N-termini of subunits a* and c* with BindN (29) and Phyre2 (30). Amino acids predicted to be RNA-binding motifs (stars) and those mutated in the present study (boxed) are shown. (C) Sequence alignment logo (31) for the predicted HLH motif in eIF3 subunit a in metazoan eIF3s, and logo for the predicted HLH motif in eIF3 subunit c in all eukaryotic eIF3s. Asterisks indicate insertions relative to the human sequence. Numbering is also according to the human sequence.
Figure 2.
Determination of the critical residues in eIF3 subunits a and c subunits important for interaction with the HCV IRES. (A) Native agarose gel showing binding of the eIF3 8-mer and its truncated versions (8-At, 8-Ct and 8-AtCt) to the full-length HCV IRES. (B) Native agarose gel showing binding to the full-length HCV IRES of the eIF3 8-mer, 8-mer with mutations in the subunit c HLH motif and/or 8-mer with mutations in the subunit a HLH motif (8-Am, 8-Cm and 8-AmCm, respectively), the 9-mer double-mutant (9-AmCm) and the 10-mer double-mutant (10-AmCm). (C) Native agarose gel showing binding of the eIF3 subunit c* and a*c* dimer to the HCV IRES IIIabc domain. The schemes of c*302 and its mutants and a*c*302 and its mutants are drawn on the right side of the corresponding panels. (D) Native agarose gel showing binding of wild-type and mutant eIF3 12-mer (12-Am, 12-Cm and 12-AmCm) to the full-length HCV IRES. Concentrations of native human eIF3, 8-mer and 8-mer mutants, 9-mer and 10-mer mutants, 12-mer and 12-mer mutants are listed in nM. Lane 1 shows HCV IRES RNA or IIIabc binding to native eIF3 or 8-mer as a control. Fluorescent full-length IRES was used at 20 nM in concentration, and the reactions were carried out in the presence of 2 μM tRNA to prevent nonspecific binding.
Figure 3.
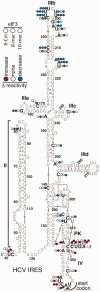
Identification of the eIF3 binding sites in the HCV IRES. SHAPE reactivity differences mapped onto the secondary structure of the IRES IIIabc domain in the presence of eIF3 8-mer mutated in the subunit c HLH motif (8-Cm), 8-mer or 10-mer, indicated from left to right by colored circles. Nucleotides in the HCV IRES that show greater (red) or reduced (blue) SHAPE reactivity in the presence of the eIF3 subcomplexes are indicated.
Figure 4.
Binding of wild-type and mutant eIF3 complexes to the 40S ribosomal subunit. Native agarose gel showing binding of the eIF3 wild-type and mutant 12-mer eIF3 to the 40S ribosomal subunit, monitored by UV absorbance of the 40S subunit rRNA. The nanomolar concentrations of the eIF3 complexes are indicated. The 40S ribosomal subunit was used at a concentration of 10 nM. The binding of the 40S subunit to natively purified human eIF3 or 12-mer is shown as a control (lane 1).
Figure 5.
Preinitiation complex formation in the presence of GST-tagged recombinant eIF3 dodecamers. (A) Sucrose gradient profile of RRL translation reaction stalled with GMPPNP and programmed with fluorescently labeled HCV IRES-containing mRNA in the presence of wild-type eIF3 dodecamer. The top of the gradient is to the left, and the absorbance at 254 nm is shown. Fractions pooled for GST affinity purification and analysis are marked 1–4. Gradients for 12-Cm and 12-Am reactions look essentially identical to that for wild-type 12-mer, due to the presence of endogenous eIF3 in the in vitro translation (IVT) reaction. (B) Analyses of GST affinity-purified products in the presence of GMPPNP. Native agarose gel of fluorescently labeled HCV IRES, western blotting of eIF2α and northern blotting of tRNAi from pooled fractions 1–4 in panel (A). (C) Sucrose gradient profile of an RRL translation reaction as in (A), but stalled with cycloheximide instead of GMPPNP. (D) Analyses of GST affinity-purified products in the presence of cycloheximide. Native agarose gel of fluorescently labeled HCV IRES, western blotting of eIF5B and northern blotting of tRNAi, from pooled fractions 1–4 in panel (C). Gradients for 12-Cm and 12-Am reactions look essentially identical to that for wild-type 12-mer, due to the presence of endogenous eIF3 in the IVT reaction. Additional western blots of GST and eIF2 and northern blots of 18S and 28S rRNA for the GMPPNP- and cycloheximide-stalled reactions are included in
Supplementary Figure S5
.
Figure 6.
The roles of the HLH motifs in eIF3 subunits a and c subunits in translation initiation complex formation. (A) Mutations in the HLH motif of eIF3a greatly weaken eIF3 binding to the HCV IRES and 40S ribosomal subunit, whereas mutations in the HLH motif of eIF3c primarily affect eIF3 binding to the 40S ribosomal subunit. (B) Mutations in the HLH motif of eIF3a disrupt eIF2-mediated Met-tRNAi loading into preinitiation complexes, whereas mutations in the eIF3c HLH motif impair eIF5B exchange with eIF2 after start codon recognition.
Similar articles
- Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit.
Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, Pestova TV, Hellen CU, Frank J. Hashem Y, et al. Nature. 2013 Nov 28;503(7477):539-43. doi: 10.1038/nature12658. Epub 2013 Nov 3. Nature. 2013. PMID: 24185006 Free PMC article. - Hepatitis C Virus Translation Regulation.
Niepmann M, Gerresheim GK. Niepmann M, et al. Int J Mol Sci. 2020 Mar 27;21(7):2328. doi: 10.3390/ijms21072328. Int J Mol Sci. 2020. PMID: 32230899 Free PMC article. Review. - Distinct regions of human eIF3 are sufficient for binding to the HCV IRES and the 40S ribosomal subunit.
Cai Q, Todorovic A, Andaya A, Gao J, Leary JA, Cate JH. Cai Q, et al. J Mol Biol. 2010 Oct 22;403(2):185-96. doi: 10.1016/j.jmb.2010.07.054. Epub 2010 Sep 15. J Mol Biol. 2010. PMID: 20816988 Free PMC article. - Coordinated assembly of human translation initiation complexes by the hepatitis C virus internal ribosome entry site RNA.
Ji H, Fraser CS, Yu Y, Leary J, Doudna JA. Ji H, et al. Proc Natl Acad Sci U S A. 2004 Dec 7;101(49):16990-5. doi: 10.1073/pnas.0407402101. Epub 2004 Nov 24. Proc Natl Acad Sci U S A. 2004. PMID: 15563596 Free PMC article. - Structural and mechanistic insights into hepatitis C viral translation initiation.
Fraser CS, Doudna JA. Fraser CS, et al. Nat Rev Microbiol. 2007 Jan;5(1):29-38. doi: 10.1038/nrmicro1558. Epub 2006 Nov 27. Nat Rev Microbiol. 2007. PMID: 17128284 Review.
Cited by
- Dynamic Interaction of Stress Granules, DDX3X, and IKK-α Mediates Multiple Functions in Hepatitis C Virus Infection.
Pène V, Li Q, Sodroski C, Hsu CS, Liang TJ. Pène V, et al. J Virol. 2015 May;89(10):5462-77. doi: 10.1128/JVI.03197-14. Epub 2015 Mar 4. J Virol. 2015. PMID: 25740981 Free PMC article. - Structure of a human 48_S_ translational initiation complex.
Brito Querido J, Sokabe M, Kraatz S, Gordiyenko Y, Skehel JM, Fraser CS, Ramakrishnan V. Brito Querido J, et al. Science. 2020 Sep 4;369(6508):1220-1227. doi: 10.1126/science.aba4904. Science. 2020. PMID: 32883864 Free PMC article. - Unlike for cellular mRNAs and other viral internal ribosome entry sites (IRESs), the eIF3 subunit e is not required for the translational activity of the HCV IRES.
Panthu B, Denolly S, Faivre-Moskalenko C, Ohlmann T, Cosset FL, Jalinot P. Panthu B, et al. J Biol Chem. 2020 Feb 14;295(7):1843-1856. doi: 10.1074/jbc.RA119.009502. Epub 2020 Jan 12. J Biol Chem. 2020. PMID: 31929110 Free PMC article. - RNA binding protein 24 regulates the translation and replication of hepatitis C virus.
Cao H, Zhao K, Yao Y, Guo J, Gao X, Yang Q, Guo M, Zhu W, Wang Y, Wu C, Chen J, Zhou Y, Hu X, Lu M, Chen X, Pei R. Cao H, et al. Protein Cell. 2018 Nov;9(11):930-944. doi: 10.1007/s13238-018-0507-x. Epub 2018 Jan 30. Protein Cell. 2018. PMID: 29380205 Free PMC article. - Structure-function analysis of the equine hepacivirus 5' untranslated region highlights the conservation of translational mechanisms across the hepaciviruses.
Lattimer J, Stewart H, Locker N, Tuplin A, Stonehouse NJ, Harris M. Lattimer J, et al. J Gen Virol. 2019 Nov;100(11):1501-1514. doi: 10.1099/jgv.0.001316. J Gen Virol. 2019. PMID: 31490115 Free PMC article.
References
- Fraser CS, Doudna JA. Structural and mechanistic insights into hepatitis C viral translation initiation. Nat. Rev. Microbiol. 2007;5:29–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases