Tet1 regulates adult hippocampal neurogenesis and cognition - PubMed (original) (raw)
. 2013 Aug 1;13(2):237-45.
doi: 10.1016/j.stem.2013.05.006. Epub 2013 Jun 13.
Qing-Yan Cui, Kiyohito Murai, Yen Ching Lim, Zachary D Smith, Shengnan Jin, Peng Ye, Luis Rosa, Yew Kok Lee, Hai-Ping Wu, Wei Liu, Zhi-Mei Xu, Lu Yang, Yu-Qiang Ding, Fuchou Tang, Alexander Meissner, Chunming Ding, Yanhong Shi, Guo-Liang Xu
Affiliations
- PMID: 23770080
- PMCID: PMC4474382
- DOI: 10.1016/j.stem.2013.05.006
Tet1 regulates adult hippocampal neurogenesis and cognition
Run-Rui Zhang et al. Cell Stem Cell. 2013.
Abstract
DNA hydroxylation catalyzed by Tet dioxygenases occurs abundantly in embryonic stem cells and neurons in mammals. However, its biological function in vivo is largely unknown. Here, we demonstrate that Tet1 plays an important role in regulating neural progenitor cell proliferation in adult mouse brain. Mice lacking Tet1 exhibit impaired hippocampal neurogenesis accompanied by poor learning and memory. In adult neural progenitor cells deficient in Tet1, a cohort of genes involved in progenitor proliferation were hypermethylated and downregulated. Our results indicate that Tet1 is positively involved in the epigenetic regulation of neural progenitor cell proliferation in the adult brain.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
Figure 1. Tet1 Deficient Mice Show Normal Brain Development but Impaired Spatial Learning and Memory
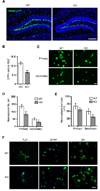
(A) Gene targeting strategy. Coding exons are shown as filled boxes and the 3’ non-coding part of exon 13 is shown as a blank box. (B) Southern blot confirmation of targeted ES clones. Fragments corresponding to the wild-type allele (wt) and the floxed allele are indicated. (C) PCR genotyping of mutant mice. Primer locations are indicated in panel A and their sequences are listed in the method. (D) Loss of Tet1 mRNA confirmed by RT-PCR assay. Gapdh was used for sample normalization. (E) – (G) Visible platform screening showed no significant differences of visual acuity and mobility between WT and the whole-body knockout (KO) mice. (E) Average swim speed. (F) Latency to the visible platform. (G) The length of swim path to the visible platform. (H) and (I) Spatial acquisition performances were recorded every day during 5-day training. (H) Escape latency. (I) Swim path to the hidden platform. (J) and (K) Probe trial for short-term memory retention was carried out 24 hours after the last training. (J) Platform crossing. (K) Time in the target quadrant (T) and other quadrants (L, R &O). (L) and (M) Probe for long-term memory retention was carried out 3 weeks after the last training. (L) Platform crossing. (M) Time in the target quadrant (T) and other quadrants (L, R &O). (N) Tet1 deficiency is compatible with brain development. Shown are Nissl-stained coronal sections from Tet1 whole-body knockout mice at postnatal day 60. Scale bar, 500 µm. For behavioral tests, 9 pairs of 4-month-old male mice were examined; For (E) – (G) and (J) – (M), two-tailed _t_-test was used in statistics; For (H) and (I), two-way ANOVA was used in statistics; * P < 0.05, **P < 0.01; All data are presented as mean ±s.e.m.
Figure 2. Reduction of _Nestin_-GFP Positive Neural Progenitor Cells in the Tet1 Deficient Dentate Gyrus and Their Impaired Proliferation in Vitro
(A) Observation of neural progenitor cells in the adult SGZ using Nestin-GFP transgenic mice. Shown are coronal images of the SGZ in 4-month-old WT (Tet1+/+; Nestin-GFP) and KO (_Tet1_−/−; Nestin-GFP) mice captured at the same exposure. Scale bar, 100 µm. (B) Quantification analysis of _Nestin_-GFP positive cells in the subgranular cell layer of dentate gyrus (n = 3 pairs of mice). (C) Isolation and culture of _Nestin_-GFP positive progenitors from 2-month-old WT and KO dentate gyrus by FACS. Scale bar, 100 µm. (D) Quantification of primary and secondary neurospheres (The initial seeding is 20,000 cells/ml, n = 3 cases). (E) Average diameters of primary and secondary neurospheres (n = 3 cases). (F) The tripotent differentiation capacity of Tet1-deficient NPCs. Neurons, astrocytes and oligodendrocytes were induced by in vitro differentiation of neurospheres isolated from WT and Tet1 KO DG. Cell lineage markers used: Tuj1 for neurons, GFAP for astrocytes and O4 for oligodendrocytes. Scale bar, 100 µm. All quantifications are presented as mean ± s.e.m. and analyzed by two-tailed _t_-test. ** P < 0.01, * P < 0.05. See also Figure S1.
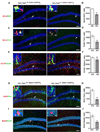
Figure 3. Decrease of Intermediate Progenitor Proliferation and Impaired Adult Neurogenesis in the Tet1 Deficient Dentate Gyrus
(A and B) Reduction of proliferating SGZ progenitors represented by BrdU & Ki67 double positive cells in adult KO (Tet1f/−; Nestin-CreERT2) mice compared to Ctrl (Tet1f/+; Nestin-CreERT2) after tamoxifen treatment (Lagace et al.) (n = 4 pairs of mice). (C and D) Reduction of BrdU+ Tbr2+ intermediate progenitors in KO compared to Ctrl after TM (n = 4 pairs of mice). (E and F) Insignificant change in the number of GFAP+ Nestin+ radial glia-like stem cells in KO compared to Ctrl after TM (n = 3 pairs of mice). (G and H) Reduction of newborn neurons represented by BrdU & Dcx double positive cells in KO compared to Ctrl after TM (n = 4 pairs of mice). (I and J) Reduction of mature neurons chased by BrdU labeling in KO compared to Ctrl after TM (n = 4 pairs of mice). All scale bars at lower right are 100 µm and the upper-left insets are enlarged images of the arrow-pointed cells with a scale bar of 10 µm. Quantifications are presented as mean ± s.e.m. and analyzed by two-tailed _t_-test. ** P < 0.01, * P < 0.05, NS, not significant. See also Figures S2 and S3.
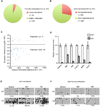
Figure 4. Promoter Hypermethylation and Down-regulation of Adult Neurogenesis-related Genes in _Nestin_-GFP Positive Progenitor Cells in Tet1 Deficient mice
_Nestin_-GFP positive progenitor cells were isolated from the DG of male adult WT (Tet1+/+; Nestin-GFP) and KO (Tet1−/−; Nestin-GFP) mice. (A) Pie representation of promoters with altered DNA methylation in Tet1 KO progenitors. A promoter was defined as −1000 to 500-bp relative to a transcription start site. The promoter methylation level was determined by calculating the average methylation level of all individual CpGs with ≥ 10 sequencing depth. A promoter was considered as differentially methylated if the absolute methylation level difference was ≥ 20% between KO and WT. (B) Pie representation of genes with expression changes in Tet1 KO progenitors. A significant expression change was defined as 1) expression ratio between KO and WT was either ≥ 2 or ≤0.5; and 2) P < 0.01 (Fisher exact test adjusted by the Benjamini-Hochberg method). (C) Scatter plot of genes with both promoter hypermethylation and significant expression changes in the KO progenitors. X-axis is the absolute difference of the methylation level (KO minus WT). Y-axis represents expression difference between KO and WT by log2 transformation of the expression ratios (KO / WT). (D) Confirmation of reduced expression of adult neurogenesis-related genes in Tet1 KO progenitors. The mRNA levels were determined by RT-qPCR. Data are normalized to Gapdh. Error bars are presented as mean ± s.e.m. (n = 6 pairs of mice). (E) Confirmation of increased promoter methylation at the Galanin, Ng2 and Ngb genes in Tet1 KO progenitors by gene specific bisulfite sequencing (n = 6 pairs of mice). Open and filled circles represent unmethylated and methylated (hydroxymethylated) CpG sites respectively. (F) 5hmC (filled circles) profiles of the Galanin, Ng2 and Ngb promoters in Tet1 KO progenitors determined by Tet-assisted bisulfite sequencing analysis (n = 6 pairs of mice). See also Table S1 and Figure S4.
Comment in
- On your (methyl) mark, get TET1, go!
Mukherjee S, Hsieh J. Mukherjee S, et al. Cell Stem Cell. 2013 Aug 1;13(2):133-4. doi: 10.1016/j.stem.2013.07.011. Cell Stem Cell. 2013. PMID: 23910076
Similar articles
- On your (methyl) mark, get TET1, go!
Mukherjee S, Hsieh J. Mukherjee S, et al. Cell Stem Cell. 2013 Aug 1;13(2):133-4. doi: 10.1016/j.stem.2013.07.011. Cell Stem Cell. 2013. PMID: 23910076 - Fetal growth restriction impairs hippocampal neurogenesis and cognition via Tet1 in offspring.
Chen W, Liu N, Shen S, Zhu W, Qiao J, Chang S, Dong J, Bai M, Ma L, Wang S, Jia W, Guo X, Li A, Xi J, Jiang C, Kang J. Chen W, et al. Cell Rep. 2021 Nov 2;37(5):109912. doi: 10.1016/j.celrep.2021.109912. Cell Rep. 2021. PMID: 34731622 - Ten-Eleven Translocation 1 and 2 Confer Overlapping Transcriptional Programs for the Proliferation of Cultured Adult Neural Stem Cells.
Shimozaki K. Shimozaki K. Cell Mol Neurobiol. 2017 Aug;37(6):995-1008. doi: 10.1007/s10571-016-0432-6. Epub 2016 Oct 24. Cell Mol Neurobiol. 2017. PMID: 27778125 Free PMC article. - Adult neurogenesis in the mammalian dentate gyrus.
Abbott LC, Nigussie F. Abbott LC, et al. Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review. - Epigenetic regulation of neurogenesis in the adult hippocampus.
Covic M, Karaca E, Lie DC. Covic M, et al. Heredity (Edinb). 2010 Jul;105(1):122-34. doi: 10.1038/hdy.2010.27. Epub 2010 Mar 24. Heredity (Edinb). 2010. PMID: 20332807 Review.
Cited by
- Physiological and pathological implications of 5-hydroxymethylcytosine in diseases.
Liang J, Yang F, Zhao L, Bi C, Cai B. Liang J, et al. Oncotarget. 2016 Jul 26;7(30):48813-48831. doi: 10.18632/oncotarget.9281. Oncotarget. 2016. PMID: 27183914 Free PMC article. Review. - Immune Regulator MCPIP1 Modulates TET Expression during Early Neocortical Development.
Jiang H, Lv X, Lei X, Yang Y, Yang X, Jiao J. Jiang H, et al. Stem Cell Reports. 2016 Sep 13;7(3):439-453. doi: 10.1016/j.stemcr.2016.07.011. Epub 2016 Aug 11. Stem Cell Reports. 2016. PMID: 27523618 Free PMC article. - The roles of epigenetic modifications in neurodegenerative diseases.
Qu W, Zhuang Y, Li X. Qu W, et al. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021 Oct 25;50(5):642-650. doi: 10.3724/zdxbyxb-2021-0160. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34986527 Free PMC article. English. - Epigenetic regulation in the neurogenic niche of the adult dentate gyrus.
Sheehy RN, Quintanilla LJ, Song J. Sheehy RN, et al. Neurosci Lett. 2022 Jan 1;766:136343. doi: 10.1016/j.neulet.2021.136343. Epub 2021 Nov 11. Neurosci Lett. 2022. PMID: 34774980 Free PMC article. Review. - Development and epigenetic plasticity of murine Müller glia.
Dvoriantchikova G, Seemungal RJ, Ivanov D. Dvoriantchikova G, et al. Biochim Biophys Acta Mol Cell Res. 2019 Oct;1866(10):1584-1594. doi: 10.1016/j.bbamcr.2019.06.019. Epub 2019 Jul 2. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31276697 Free PMC article.
References
- Abbosh C, Lawkowski A, Zaben M, Gray W. GalR2/3 mediates proliferative and trophic effects of galanin on postnatal hippocampal precursors. J Neurochem. 2011;117:425–436. - PubMed
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nature reviews Genetics. 2012;13:7–13. - PubMed
- Brewer GJ, Torricelli JR. Isolation and culture of adult neurons and neurospheres. Nature protocols. 2007;2:1490–1498. - PubMed
- Burmester T, Weich B, Reinhardt S, Hankeln T. A vertebrate globin expressed in the brain. Nature. 2000;407:520–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA087869/CA/NCI NIH HHS/United States
- R01 HD045022/HD/NICHD NIH HHS/United States
- R01 NS059546/NS/NINDS NIH HHS/United States
- RC1 NS068370/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases