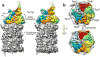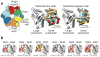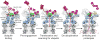Conformational switching of the 26S proteasome enables substrate degradation - PubMed (original) (raw)
Conformational switching of the 26S proteasome enables substrate degradation
Mary E Matyskiela et al. Nat Struct Mol Biol. 2013 Jul.
Abstract
The 26S proteasome is the major eukaryotic ATP-dependent protease, responsible for regulating the proteome through degradation of ubiquitin-tagged substrates. Its regulatory particle, containing the heterohexameric AAA+ ATPase motor and the essential deubiquitinase Rpn11, recognizes substrates, removes their ubiquitin chains and translocates them into the associated peptidase after unfolding, but detailed mechanisms remain unknown. Here we present the 26S proteasome structure from Saccharomyces cerevisiae during substrate degradation, showing that the regulatory particle switches from a preengaged to a translocation-competent conformation. This conformation is characterized by a rearranged ATPase ring with uniform subunit interfaces, a widened central channel coaxially aligned with the peptidase and a spiral orientation of pore loops that suggests a rapid progression of ATP-hydrolysis events around the ring. Notably, Rpn11 moves from an occluded position to directly above the central pore, thus facilitating substrate deubiquitination concomitant with translocation.
Figures
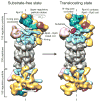
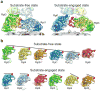
Figure 1. Conformational transition of the proteasome from a substrate-free to an actively degrading state
The structures of wild-type proteasome in its substrate-free (left) and substrate-engaged state (right) identically oriented based on their 20S peptidase (grey), with a dashed line indicating the central axis of the peptidase pore. Substrate engagement induces a conformational rearrangement of the regulatory particle, including a rotation of Rpn2 (dark blue), Rpn13 (light orange), and the lid subcomplex (yellow), the formation of contacts between the ubiquitin receptor Rpn10 (purple) and the Rpt4–Rpt5 coiled coil, and a coaxial alignment of the N-ring and the AAA+ ring (both cyan) with the peptidase. Furthermore, the DUB Rpn11 (green) shifts to a central location, occluding the processing pore. The extra density (red) observed in the reconstruction of the degrading proteasome is attributed to a globular domain of the substrate.
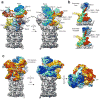
Figure 2. The subnanometer-resolution structure of the substrate-engaged 26S proteasome
(a) The segmented cryo-EM reconstruction of the substrate-engaged proteasome (Rpn11AXA Rpn13Δ), with the regulatory particle colored by subunit and the peptidase in grey. (b) Side views of the base subcomplex in the substrate-free (top) and substrate-bound state (bottom), emphasizing the substrate-induced twisting of the Rpt3–Rpt6 coiled coil (green/red) that results in a rotation of Rpn2 (blue). The core-particle densities were aligned for this comparison. (c) The motions associated with substrate engagement are depicted by overlaying the substrate-free and substrate-bound structures that are aligned by their 20S peptidases. The base (apo as blue mesh, substrate-bound as solid cyan) and the lid (apo as red mesh, substrate-bound as solid yellow) undergo large rotations and shifts, while the peptidase (apo as black mesh, substrate-bound as solid grey) does not exhibit notable differences. On the left, the red and yellow curved lines illustrate the movement of the horseshoe-shaped arrangement of PCI domains from its substrate-free to substrate-bound position, respectively. On the right, a top view illustrates the 25° rotation of the upper regulatory particle around the axis of the Rpt3–Rpt6 coiled coil (black circle).
Figure 3. Rpn11 is coaxially aligned with the ATPase pore in the substrate-engaged state
(a) An atomic model of the DUB Rpn11 (PDB ID: 4B4T) is used to show the substrate-induced movement of this subunit relative to the N-ring. In the substrate-free state, Rpn11 (semi-transparent green ribbon), with the residues predicted to form the catalytic groove highlighted in orange, is situated to the side of the N-ring and behind the Rpt4–Rpt5 coiled coil. Conformational changes in the regulatory particle shift Rpn11 to a position directly above the N-ring pore in the substrate-bound state (opaque ribbon). (b) The expected orientation of a ubiquitin moiety (purple ribbon) with its C-terminus bound in the Rpn11 catalytic groove (Rpn11 electron density in green mesh, atomic model in green ribbon with catalytic groove highlighted in orange) is shown from a side and top view. c, Close-up of the modeled interactions between ubiquitin and Rpn11 from the top view. The continuous density closing the catalytic groove (purple mesh) may correspond to the C-terminus of ubiquitin.
Figure 4. Substrate-induced rearrangement of the ATPase subunits creates a widened pore and a continuous central channel throughout the enzyme
(a) The segmented electron densities corresponding to the ATPase subunits Rpt1-Rpt6 (rainbow) and the peptidase (grey) are shown for the proteasome in the absence (left) and presence (right) of substrate, with dotted lines indicating the axes of the central channels. Substrate-engagement causes the AAA+ domains of the Rpts to individually rotate and shift into a more symmetric and coaxially-aligned ring. The N-ring also tilts and shifts, and together these changes result in the formation of a continuous channel through the ATPases to the peptidase. (b) The peptidase (grey) and the AAA+ domains of the ATPases (rainbow) are shown from above in the absence (top) and presence (bottom) of substrate, with dashed black lines indicating the 7-fold symmetry of the peptidase below. The large white circles encompassing the AAA+ domains emphasize the degree of alignment between the AAA+ ring and the peptidase. The smaller white circles depict the ATPase-pore diameters for the two states.
Figure 5. Bi-modal stabilization of the pre-engaged or translocation-competent base conformation by the lid
Close-up view of the lid-base interface highlights alternative contacts between Rpt and Rpn subunits in the substrate-free and substrate-engaged conformations of the regulatory particle. The positions of Rpt3 (green), Rpt4 (yellow), and Rpt6 (red) within the substrate-free and substrate-engaged EM densities (grey mesh) are shown using fitted crystal structures of the homologous PAN AAA+ domain (PDB ID: 3H4M). The crystal structure of Rpn6 (cyan, PDB ID: 3TXN ) and homology models of Rpn5 (PDB ID: 4B4T, light yellow) and Rpn7 (PDB ID: 4B4T, purple) are shown on the right and docked into their corresponding positions in the EM density (middle and left). Both Rpn5 and Rpn6 interact with the small AAA+ subdomain of Rpt3, while Rpn7 contacts the interface between the small AAA+ subdomain of Rpt6 and the large AAA+ subdomain of Rpt3. These interactions in the substrate-free state are highlighted with solid blue circles. The substrate-engaged reconstruction reveals that Rpt3 switches its contacts with Rpn5 and Rpn6 to new binding sites (solid red circles) that are located 30 and 25 Å further toward the respective PCI domains. In contrast, Rpn7 remains in contact with the Rpt6/3 interface, but reduces its interaction points from two (blue circles) to one (red circle). This semi-static joint with Rpn7 may function as a pivot point in switching from a substrate-free to a substrate-bound conformation of the regulatory particle. Dashed circles indicate the corresponding contacts in the alternative conformation.
Figure 6. The translocation-competent conformation of the base exhibits uniform AAA+ domain interfaces
(a) On the left, a cartoon with subunits individually colored delineates the inter-subunit “rigid body” (dashed line) formed from a small AAA+ subdomain and the large AAA+ subdomain of its counterclockwise neighbor ,. The six “rigid bodies” derived from docked crystal structures of individual large and small AAA+ subdomains of the homologous PAN (PDB ID: 3H4M) were superimposed by aligning the large subdomains. Substrate engagement induces uniform interfaces between subdomains of neighboring subunits, reflected by a lower average RMSD of the small subdomains. (b) “Rigid bodies” formed between large and small AAA+ subdomains at each Rpt interface in the absence and presence of substrate are superimposed and aligned by their large subdomain (grey). The small AAA+ domains are shown individually colored in the substrate-free state and magenta in the bound state.
Figure 7. Rearrangement of the spiral staircase upon substrate engagement
(a) Cutaway side view of the Rpt ring in the substrate-free (left) and substrate-engaged (right) state, with Rpt6 and Rpt5 removed for clarity, respectively, and oriented with the top subunit of each spiral staircase on the left. Individually docked copies of the PAN crystal structure (ribbons, PDB ID: 3H4M) reveal different spiral staircase arrangements in the two states, which are emphasized by a sphere representation of the pore-loop residue that is predicted to drive translocation. (b) The AAA+ domains of Rpt1–Rpt6 are shown individually in the same orientation, with their pore loops facing right and the aromatic pore-loop residue shown in purple. In the absence of substrate, the entire AAA+ domains are rotated to varying degrees away from the central pore, leading to a pronounced spiral-staircase arrangement of large subdomains with a global pitch that is indicated by a continuous line. Substrate engagement arranges the AAA+ domains at a more uniform height, with a lower-pitch spiral staircase of pore loops established solely through varied tilting of the large subdomains (emphasized by black lines).
Figure 8. Structure-based model for substrate engagement and degradation by the 26S proteasome
Cutaway side view of the proteasome reconstructions in the substrate-free and engaged conformations. In the first step, substrate (red) is tethered through its ubiquitin chain (purple) to the UIM of Rpn10 (yellow cylinder). In this pre-engaged state, the flexible substrate tail can enter the accessible N-ring pore and contact the uppermost subunits of the AAA+ domain spiral staircase. Upon substrate engagement, the Rpts become rearranged into a new spiral staircase with a widened central pore that is aligned with the N-ring and subjacent peptidase (grey). Concomitantly, Rpn11 (green) shifts to a central location directly above the N-ring pore, exposing its active site (pink dot) for ubiquitin scanning along the translocating polypeptide. All ubiquitin modifications are removed as their isopeptide attachment site (yellow dot) passes by Rpn11, facilitating fast translocation, unfolding, and degradation of the substrate.
Similar articles
- An AAA Motor-Driven Mechanical Switch in Rpn11 Controls Deubiquitination at the 26S Proteasome.
Worden EJ, Dong KC, Martin A. Worden EJ, et al. Mol Cell. 2017 Sep 7;67(5):799-811.e8. doi: 10.1016/j.molcel.2017.07.023. Epub 2017 Aug 24. Mol Cell. 2017. PMID: 28844860 - Structure of an endogenous yeast 26S proteasome reveals two major conformational states.
Luan B, Huang X, Wu J, Mei Z, Wang Y, Xue X, Yan C, Wang J, Finley DJ, Shi Y, Wang F. Luan B, et al. Proc Natl Acad Sci U S A. 2016 Mar 8;113(10):2642-7. doi: 10.1073/pnas.1601561113. Epub 2016 Feb 29. Proc Natl Acad Sci U S A. 2016. PMID: 26929360 Free PMC article. - Substrate-engaged 26_S_ proteasome structures reveal mechanisms for ATP-hydrolysis-driven translocation.
de la Peña AH, Goodall EA, Gates SN, Lander GC, Martin A. de la Peña AH, et al. Science. 2018 Nov 30;362(6418):eaav0725. doi: 10.1126/science.aav0725. Epub 2018 Oct 11. Science. 2018. PMID: 30309908 Free PMC article. - Structure and Function of the 26S Proteasome.
Bard JAM, Goodall EA, Greene ER, Jonsson E, Dong KC, Martin A. Bard JAM, et al. Annu Rev Biochem. 2018 Jun 20;87:697-724. doi: 10.1146/annurev-biochem-062917-011931. Epub 2018 Apr 13. Annu Rev Biochem. 2018. PMID: 29652515 Free PMC article. Review. - Structure, Dynamics and Function of the 26S Proteasome.
Mao Y. Mao Y. Subcell Biochem. 2021;96:1-151. doi: 10.1007/978-3-030-58971-4_1. Subcell Biochem. 2021. PMID: 33252727 Review.
Cited by
- The Cryo-EM Effect: Structural Biology of Neurodegenerative Disease Proteostasis Factors.
Creekmore BC, Chang YW, Lee EB. Creekmore BC, et al. J Neuropathol Exp Neurol. 2021 Jun 4;80(6):494-513. doi: 10.1093/jnen/nlab029. J Neuropathol Exp Neurol. 2021. PMID: 33860329 Free PMC article. Review. - Tuning the proteasome to brighten the end of the journey.
Mayor T, Sharon M, Glickman MH. Mayor T, et al. Am J Physiol Cell Physiol. 2016 Nov 1;311(5):C793-C804. doi: 10.1152/ajpcell.00198.2016. Epub 2016 Sep 7. Am J Physiol Cell Physiol. 2016. PMID: 27605452 Free PMC article. Review. - Measurement of the Multiple Activities of 26S Proteasomes.
Kim HT, Collins GA, Goldberg AL. Kim HT, et al. Methods Mol Biol. 2018;1844:289-308. doi: 10.1007/978-1-4939-8706-1_19. Methods Mol Biol. 2018. PMID: 30242717 Free PMC article. - Structure of the Cdc48 ATPase with its ubiquitin-binding cofactor Ufd1-Npl4.
Bodnar NO, Kim KH, Ji Z, Wales TE, Svetlov V, Nudler E, Engen JR, Walz T, Rapoport TA. Bodnar NO, et al. Nat Struct Mol Biol. 2018 Jul;25(7):616-622. doi: 10.1038/s41594-018-0085-x. Epub 2018 Jul 2. Nat Struct Mol Biol. 2018. PMID: 29967539 Free PMC article. - Crystal structure of the human COP9 signalosome.
Lingaraju GM, Bunker RD, Cavadini S, Hess D, Hassiepen U, Renatus M, Fischer ES, Thomä NH. Lingaraju GM, et al. Nature. 2014 Aug 14;512(7513):161-5. doi: 10.1038/nature13566. Epub 2014 Jul 16. Nature. 2014. PMID: 25043011
References
- Sauer RT, Baker TA. AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem. 2011;80:587–612. - PubMed
- Saeki Y, Tanaka K. Assembly and function of the proteasome. Methods Mol Biol. 2012;832:315–37. - PubMed
- Groll M, et al. A gated channel into the proteasome core particle. Nat Struct Biol. 2000;7:1062–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases