Immune activation and HIV persistence: implications for curative approaches to HIV infection - PubMed (original) (raw)
Review
Immune activation and HIV persistence: implications for curative approaches to HIV infection
Nichole R Klatt et al. Immunol Rev. 2013 Jul.
Abstract
Despite complete or near-complete suppression of human immunodeficiency virus (HIV) replication with combination antiretroviral therapy, both HIV and chronic inflammation/immune dysfunction persist indefinitely. Untangling the association between the virus and the host immune environment during therapy might lead to novel interventions aimed at either curing the infection or preventing the development of inflammation-associated end-organ disease. Chronic inflammation and immune dysfunction might lead to HIV persistence by causing virus production, generating new target cells, enabling infecting of activated and resting target cells, altering the migration patterns of susceptible target cells, increasing the proliferation of infected cells, and preventing normal HIV-specific clearance mechanisms from function. Chronic HIV production or replication might contribute to persistent inflammation and immune dysfunction. The rapidly evolving data on these issues strongly suggest that a vicious cycle might exist in which HIV persistence causes inflammation that in turn contributes to HIV persistence.
© 2013 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
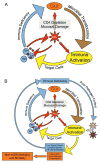
Fig. 1. HIV initiates and sustains a ‘vicious cycle’
(A). Acute HIV infection causes damage to the mucosal integrity of the gastrointestinal tract, resulting in continuous local and systemic exposure to gut microbial products. HIV and these microbial products cause activation of T cells and expansion of CD4+ T cells, resulting in more target cells and higher levels of HIV replication. Direct and indirect mechanisms lead to CD4+ T-cell loss, broad immunodeficiency, and higher levels of both HIV and microbial translocation. (B). Chronic activation of the immune system results in direct damage to lymphoid tissues, which in turn contributes to failure to regenerate T cells and overall decrease in function of adaptive and innate immune systems. The resulting immunodeficiency results in excess pathogens (e.g. HIV, gut microbes, herpes viruses) and, as a result, yet more immune activation. This chronic inflammatory state predicts and presumably causes development of AIDS and non-AIDS conditions such as early cardiovascular disease.
Fig. 2. Immune activation sustains HIV persistence during antiretroviral therapy
HIV-associated damage to the lymphoid system is only partially reversible. During long-term antiretroviral therapy, the residual immune dysfunction (which is due in part to loss of thymic tissue, fibrosis in germinal centers of secondary lymphoid structure, hematopoietic stem cell loss, and mucosal barrier breakdown) results in immunodeficiency, excess amounts of pathogens and chronic immune activation. The immune activation in turn leads to migration of CD4+ T cells to foci of HIV replication, generation of activated and susceptible target cells, and production of virus from latently infected cells (all of which enable more efficient cell-to-cell spread of HIV and replenishment of infected cells). The inflammatory environment also leads to proliferation and maintenance of latently infected cells. The residual HIV replication/production in turn contributes to sustained tissue damage and immune activation.
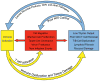
Fig. 3. Mechanisms by which immune activation causes HIV persistence
The chronic immune dysfunction of antiretroviral-treated HIV infection contributes to HIV persistence by (1) enabling HIV replication via generation of activated CD4+ T cells, (2) enabling infection of resting cells, (3) reducing the capacity of the adaptive immune system to clear infected cells, (4) causing differentiation and proliferation of infected cells, and (5) increasing expression of cell-surface negative regulators, which in turn contributes to persistence of latently infected cells. Detailed knowledge regarding the mechanisms which contributes to each of these steps might lead to the development of immune-based therapeutics which could contribute to an HIV cure.
Similar articles
- Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.
Pierson T, McArthur J, Siliciano RF. Pierson T, et al. Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review. - Short Intracellular HIV-1 Transcripts as Biomarkers of Residual Immune Activation in Patients on Antiretroviral Therapy.
Ishizaka A, Sato H, Nakamura H, Koga M, Kikuchi T, Hosoya N, Koibuchi T, Nomoto A, Kawana-Tachikawa A, Mizutani T. Ishizaka A, et al. J Virol. 2016 May 27;90(12):5665-5676. doi: 10.1128/JVI.03158-15. Print 2016 Jun 15. J Virol. 2016. PMID: 27030274 Free PMC article. - Persisting inflammation and chronic immune activation but intact cognitive function in HIV-infected patients after long-term treatment with combination antiretroviral therapy.
Pedersen KK, Pedersen M, Gaardbo JC, Ronit A, Hartling HJ, Bruunsgaard H, Gerstoft J, Ullum H, Nielsen SD. Pedersen KK, et al. J Acquir Immune Defic Syndr. 2013 Jul 1;63(3):272-9. doi: 10.1097/QAI.0b013e318289bced. J Acquir Immune Defic Syndr. 2013. PMID: 23392469 - Immune activation and HIV persistence: considerations for novel therapeutic interventions.
Hatano H. Hatano H. Curr Opin HIV AIDS. 2013 May;8(3):211-6. doi: 10.1097/COH.0b013e32835f9788. Curr Opin HIV AIDS. 2013. PMID: 23454864 Free PMC article. Review. - Monocytes as regulators of inflammation and HIV-related comorbidities during cART.
Anzinger JJ, Butterfield TR, Angelovich TA, Crowe SM, Palmer CS. Anzinger JJ, et al. J Immunol Res. 2014;2014:569819. doi: 10.1155/2014/569819. Epub 2014 Jun 12. J Immunol Res. 2014. PMID: 25025081 Free PMC article. Review.
Cited by
- PET Imaging Reveals Early Pulmonary Perfusion Abnormalities in HIV Infection Similar to Smoking.
Kohli P, Kelly VJ, Hibbert KA, Corleis B, Kone M, Cho JL, DeFaria-Yeh D, Kwon DS, Medoff BD, Harris RS, Winkler T. Kohli P, et al. J Nucl Med. 2021 Mar;62(3):405-411. doi: 10.2967/jnumed.120.245977. Epub 2020 Aug 6. J Nucl Med. 2021. PMID: 32764123 Free PMC article. - (1→3)-β-d-Glucan: A Biomarker for Microbial Translocation in Individuals with Acute or Early HIV Infection?
Hoenigl M, Pérez-Santiago J, Nakazawa M, de Oliveira MF, Zhang Y, Finkelman MA, Letendre S, Smith D, Gianella S. Hoenigl M, et al. Front Immunol. 2016 Oct 3;7:404. doi: 10.3389/fimmu.2016.00404. eCollection 2016. Front Immunol. 2016. PMID: 27752257 Free PMC article. - Depletion and activation of mucosal CD4 T cells in HIV infected women with HPV-associated lesions of the cervix uteri.
Mbuya W, Mcharo R, Mhizde J, Mnkai J, Mahenge A, Mwakatima M, Mwalongo W, Chiwerengo N, Hölscher M, Lennemann T, Saathoff E, Rwegoshora F, Torres L, Kroidl A, Geldmacher C, Held K, Chachage M. Mbuya W, et al. PLoS One. 2020 Oct 2;15(10):e0240154. doi: 10.1371/journal.pone.0240154. eCollection 2020. PLoS One. 2020. PMID: 33007050 Free PMC article. - Complement opsonization of HIV affects primary infection of human colorectal mucosa and subsequent activation of T cells.
Bhattacharya P, Ellegård R, Khalid M, Svanberg C, Govender M, Keita ÅV, Söderholm JD, Myrelid P, Shankar EM, Nyström S, Larsson M. Bhattacharya P, et al. Elife. 2020 Sep 2;9:e57869. doi: 10.7554/eLife.57869. Elife. 2020. PMID: 32876566 Free PMC article.
References
- Valdez H, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–1866. - PubMed
- Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS Rev. 2006;8:88–97. - PubMed
- French MA, King MS, Tschampa JM, da Silva BA, Landay AL. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J Infect Dis. 2009;200:1212–1215. - PubMed
- Hunt PW, et al. T Cell Activation Is Associated with Lower CD4+ T Cell Gains in Human Immunodeficiency Virus-Infected Patients with Sustained Viral Suppression during Antiretroviral Therapy. J Infect Dis. 2003;187:1534–1543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U19AI096109/AI/NIAID NIH HHS/United States
- U19 AI096109/AI/NIAID NIH HHS/United States
- K24 AI069994/AI/NIAID NIH HHS/United States
- R01 AI087145/AI/NIAID NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical