Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss - PubMed (original) (raw)
. 2013 Jul 2;110(27):E2518-27.
doi: 10.1073/pnas.1306832110. Epub 2013 Jun 17.
Sara Sanz-Blasco, Xiaofei Zhang, Peng Xia, Mohd Waseem Akhtar, Shu-ichi Okamoto, Gustavo Dziewczapolski, Tomohiro Nakamura, Gang Cao, Alexander E Pratt, Yeon-Joo Kang, Shichun Tu, Elena Molokanova, Scott R McKercher, Samuel Andrew Hires, Hagit Sason, David G Stouffer, Matthew W Buczynski, James P Solomon, Sarah Michael, Evan T Powers, Jeffery W Kelly, Amanda Roberts, Gary Tong, Traci Fang-Newmeyer, James Parker, Emily A Holland, Dongxian Zhang, Nobuki Nakanishi, H-S Vincent Chen, Herman Wolosker, Yuqiang Wang, Loren H Parsons, Rajesh Ambasudhan, Eliezer Masliah, Stephen F Heinemann, Juan C Piña-Crespo, Stuart A Lipton
Affiliations
- PMID: 23776240
- PMCID: PMC3704025
- DOI: 10.1073/pnas.1306832110
Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss
Maria Talantova et al. Proc Natl Acad Sci U S A. 2013.
Erratum in
- Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13691
- Correction for Talantova et al., Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss.
[No authors listed] [No authors listed] Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):E3630. doi: 10.1073/pnas.1511280112. Epub 2015 Jun 17. Proc Natl Acad Sci U S A. 2015. PMID: 26085137 Free PMC article. No abstract available. - Correction to Supporting Information for Talantova et al., Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss.
[No authors listed] [No authors listed] Proc Natl Acad Sci U S A. 2015 Jul 14;112(28):E3751-2. doi: 10.1073/pnas.1511282112. Epub 2015 Jun 23. Proc Natl Acad Sci U S A. 2015. PMID: 26106153 Free PMC article. No abstract available.
Abstract
Synaptic loss is the cardinal feature linking neuropathology to cognitive decline in Alzheimer's disease (AD). However, the mechanism of synaptic damage remains incompletely understood. Here, using FRET-based glutamate sensor imaging, we show that amyloid-β peptide (Aβ) engages α7 nicotinic acetylcholine receptors to induce release of astrocytic glutamate, which in turn activates extrasynaptic NMDA receptors (eNMDARs) on neurons. In hippocampal autapses, this eNMDAR activity is followed by reduction in evoked and miniature excitatory postsynaptic currents (mEPSCs). Decreased mEPSC frequency may reflect early synaptic injury because of concurrent eNMDAR-mediated NO production, tau phosphorylation, and caspase-3 activation, each of which is implicated in spine loss. In hippocampal slices, oligomeric Aβ induces eNMDAR-mediated synaptic depression. In AD-transgenic mice compared with wild type, whole-cell recordings revealed excessive tonic eNMDAR activity accompanied by eNMDAR-sensitive loss of mEPSCs. Importantly, the improved NMDAR antagonist NitroMemantine, which selectively inhibits extrasynaptic over physiological synaptic NMDAR activity, protects synapses from Aβ-induced damage both in vitro and in vivo.
Keywords: astrocytes; glutamate receptors; α7-nicotinics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
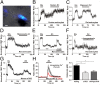
Fig. 1.
Detection of astrocytic glutamate release after exposure to oligomeric Aβ. (A) Coculture of purified rat cortical astrocytes and HEK293T cells cotransfected with SuperGluSnFR and neuroligin to measure directly the time course of glutamate release. FRET fluorescence overlaid on bright-field imaging. (Scale bar: 10 µm.) (B) Human naturally occurring Aβ peptide (55 pM by ELISA; Materials and Methods) was applied to a coculture of purified human astrocytes and HEK cells expressing SuperGluSnFR, and the normalized FRET ratio was measured. The peak CFP/YFP ratio was divided by the baseline CFP/YFP ratio and was plotted after baseline normalization to 1. As measured with the FRET probe, Aβ induced glutamate (Glu) release from human astrocytes comparable to control applications of glutamate of ∼30 µM. (C) Normalized FRET ratio reflecting glutamate release from purified rat astrocytes exposed to synthetic Aβ1–42 (containing 250-nM oligomers; Materials and Methods). (D) Monomeric Aβ1–42 (1 µM) did not induce glutamate release from purified astrocytes. Glutamate addition was used as a control. (E) Oligomerized Aβ1–42 generated a robust FRET signal from astrocyte cultures in the presence but not the absence of extracellular Ca2+. n = 24 cells analyzed in four experiments. (F) α-Bungarotoxin (100 nM), a selective antagonist of α7 nAChRs, abrogated oligomerized Aβ1–42-induced glutamate release from rat astrocytes. n = 14 cells analyzed in three experiments. (G) Oligomerized Aβ1–42-induced glutamate release also was largely eliminated in astrocytes from α7nAChR-knockout (α7KO) mice. n = 25 cells analyzed in four experiments. Values of the normalized FRET ratio in each panel are mean ± SEM. (H) By Fura-2 imaging, oligomerized Aβ1–42 evoked a larger increase in intracellular Ca2+ in WT than in α7KO mouse astrocytes. n = 83 cells analyzed in three experiments. (I) In vivo microdialysis showed higher levels of extracellular glutamate in the hippocampus of 22- to 24-mo-old transgenic mice overexpressing human APP (hAPP tg) than in age-matched α7KO mice or in mice produced by crossing hAPP tg mice with α7KO mice (hAPP tg/α7KO). Data are shown as mean + SEM; n = 16; *P ≤ 0.05 by t test with Bonferroni correction.
Fig. 2.
Application of various Aβ preparations (naturally occurring human Aβ, oligomeric synthetic Aβ1–42, or Aβ25–35) to autaptic hippocampal neuronal cultures induces extrasynaptic inward currents and decreases mEPSC frequency in a glutamate receptor antagonist-sensitive manner. (A) Naturally occurring human Aβ (55 pM) induced extrasynaptic current in neurons that was inhibited by glutamate receptor antagonists NBQX (10 µM) and
d
-APV (100 µM). (B) Aβ1–42 (containing 500-nM oligomers) induced extrasynaptic current in glutamatergic autaptic neurons, which could be largely inhibited by NBQX (10 µM) plus memantine (10 µM). (C) NBQX (10 µM) plus memantine (10 µM) significantly reduced the amplitude of Aβ1–42-induced extrasynaptic currents. Data are shown as mean + SEM; n = 8; *P < 0.05 by t test. (D) Oligomerized Aβ1–42 also induced extrasynaptic current in GABAergic autaptic neurons. Large, transient inward current represents an inhibitory postsynaptic current. (E_–_G) Application of Aβ25–35 (10 µM), but not Aβ35–25, also induced inward extrasynaptic current sensitive to memantine with a mean amplitude of 45.9 ± 11.2 pA in 42% of recorded cells. (H) In well space-clamped autapses, both mEPSC amplitude and mEPSC frequency were decreased significantly after exposure to oligomerized Aβ1–42. n = 9; *P < 0.05, **P < 0.01 by t test. (I) Representative cumulative probability graphs of mEPSC amplitudes. (J) Representative cumulative probability graphs of mEPSC interevent intervals. (K) Amplitude of mEPSC was not altered but frequency was decreased significantly after Aβ25–25 exposure. n = 5; *P < 0.05 by t test. (L) Representative cumulative probability graphs of mEPSC amplitudes. (M) Representative cumulative probability graphs of mEPSC interevent intervals.
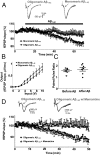
Fig. 3.
Memantine and NitroMemantine inhibit Aβ-induced [Ca2+]i increase and NO generation in cultured rat primary cortical neurons. (A) Images of cells before (Baseline) and after exposure to Aβ1–42 (250-nM oligomers) with and without treatment with memantine. Colored bar indicates neuronal Ca2+ levels ([Ca2+]i) determined with Fura-2/AM. (B and C) Change in Fura-2 and DAF fluorescence intensity with the addition of monomeric (1 µM) or oligomeric Aβ1–42 (250 nM) in the presence and absence of memantine or NitroMemantine (5 µM). Values for the change in fluorescence intensity were calculated as change in intensity divided by baseline intensity (ΔF/F0) and were plotted as a fraction of 1. Values are mean + SEM for all panels. *P < 0.05, **P < 0.01, ***P < 0.001; n ≥ 40 neurons for each condition. a.u., arbitrary units.
Fig. 4.
Soluble oligomeric Aβ1–42 induces synaptic depression in hippocampal slices. (A) fEPSPs were gradually depressed after slices were perfused with 50 nM oligomeric Aβ1–42. In contrast, monomeric Aβ1–42 (1 μM) had no effect on fEPSPs. n = 12. (B) Effect of oligomeric vs. monomeric Aβ1–42 on input–output curves. n = 12. (C) Effect of oligomeric vs. monomeric Aβ1–42 on paired-pulse ratio. n = 12. (D) Memantine inhibited oligomeric Aβ1–42-induced synaptic depression. n = 11.
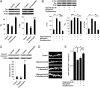
Fig. 5.
NMDAR antagonists inhibit relatively large basal glutamatergic currents (Iglu) observed in hippocampal slices from hAPP-J20 tg mice but not from WT littermates during whole-cell recording. (A) In WT mice, 100 µM CPP/50 µM CNQX blocked a small background Iglu of 9.5 pA observed at a holding potential (Vh) = −70 mV. (B) In an APP-J20 tg littermate, 100 µM CPP/50 µM CNQX blocked a larger basal Iglu of 53.6 pA at Vh = −70 mV. (C and D) In slices from both WT and J20 transgenic littermates, 100 µM CPP/50 µM CNQX also blocked mEPSCs. (Left) Untreated control. (Right) Drug treated. (E) At Vh = −70 mV, 100 µM CPP/50 µM CNQX inhibited a mean Iglu of 9.9 pA in WT littermates and 39.3 pA in transgenic littermates. n = 8; *P < 0.01. (F) In another WT littermate, there was little if any basal Iglu, and perfusion with 10 µM memantine manifested no effect at Vh = −70 mV. (G) In a J20 transgenic littermate, perfusion with 10 µM memantine blocked a background Iglu of 46.5 pA at Vh = −70 mV. (H) Memantine blocked a mean basal Iglu of 4.7 pA in hippocampal slices from WT mice but 41.2 pA in J20 transgenic littermates. n = 7; *P < 0.01. (I) In WT slices, 10 µM memantine manifested little or no effect on the frequency or amplitude of mEPSCs, even with very prolonged incubation times on the order of hours. (J) In slices from hAPP-J20 tg littermates, perfusion with 10 µM memantine for periods ≥30 min resulted in increased frequency of mEPSCs, as reflected by a leftward shift in the interevent interval in the cumulative probability curve, but had only minor or no effect on amplitude. n = 7 slices for I and J. (Insets) Histograms of frequency and amplitude. Data are shown as mean + SEM; *P < 0.01. (K) mEPSCs recorded from CA1 neurons in WT mice in the presence of 1 µM TTX and 50 µM picrotoxin (Vh = −70 mV). (L) mEPSCs recorded in hAPP-J20 tg mice under similar conditions. (M) Cumulative probability showing decreased mEPSC frequency in J20 transgenic vs. WT mice, as reflected by an increase in the interevent interval. n = 12; P < 0.00001 for mEPSC frequency by Kolmogorov–Smirnov test. The noise level was greater in J20 than in WT mice because of the presence of increased basal current from extrasynaptic glutamate. To avoid bias due to this noise level, the analysis of mEPSC frequency used the same event threshold for both sets of data. (N and O) Cumulative probability of mEPSC amplitude and kinetics in hAPP-J20 tg vs. WT mice. n = 12.
Fig. 6.
eNMDAR activity triggers the molecular cascade and dendritic spine loss associated with synaptic damage induced by Aβ peptide. (A) Western blots showing that synaptic activity reduced and extrasynaptic NMDAR activity increased the levels of tau and phospho-tau (p-tau) in mixed neuronal/glial cultures. Quantification is shown relative to the level of actin as the loading control. *P < 0.05, **_P_ < 0.01, and ***_P_ < 0.001 by ANOVA. (_B_) Blockade of extrasynaptic relative to synaptic NMDAR activity using memantine or NitroMemantine (5 μM each) decreased p-tau and to a lesser degree total tau levels after exposure to oligomerized Aβ1–42. _n_ = 3. The effect of NitroMemantine was greater than that of memantine. Quantification is shown relative to actin. *_P_ < 0.01, **_P_ < 0.001, ***_P_ < 0.05 by ANOVA. (_C_) Western blots showing that synaptic activity reduced and extrasynaptic NMDAR activity increased cleaved caspase-3. Quantification is shown relative to actin. *_P_ < 0.01, **_P_ < 0.001 by ANOVA. (_D_ and _E_) Blockade of eNMDAR activity by NitroMemantine abrogated dendritic spine loss mediated by oligomerized Aβ in hippocampal slices to a greater degree than memantine. Hippocampal neurons from YFP-transgenic mice were exposed for 7 d to control or synthetic Aβ1–42 (500-nM oligomers) in the presence or absence of memantine or NitroMemantine (each at 10 μM). _n_ > 12 for each condition; *P < 0.001, **P < 0.05. Histograms for all panels show mean + SEM.
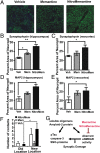
Fig. 7.
Immunocytochemical and neurobehavioral analysis of AD transgenic mice plus schematic of Aβ effects on synapses. (A–E) Quantitative confocal fluorescence imaging in hippocampus and frontal cortex of synaptic marker synaptophysin (representative images from hippocampus are shown in A) and dendritic marker MAP2 in 3× tg AD mice treated with vehicle, memantine, or NitroMemantine. n = 9; *P < 0.05, **P < 0.01. (F) Improvement in neurobehavioral assessment of hippocampal function on the location novelty recognition test [or novel location (NL)] in 9-mo-old 3× tg AD mice after a 3-mo treatment with NitroMemantine compared with the effect of memantine or vehicle-treated control. In this task, the number of contacts with the object in the final familiarization trial before the object was moved (Old Location) and the number of contacts made after the same object was moved (New Location) were monitored. There were no group differences in initial contacts with the three objects, and all were explored between five and seven times during the first familiarization trial. Only the NitroMemantine-treated group manifested a significant increase in their ability to detect spatial change, as monitored by the increased number of contacts with the object after it was moved. *P < 0.03 by ANOVA; n = 24. (G) Schematic diagram showing influence of eNMDAR activity on Aβ-induced synaptic damage in AD.
Similar articles
- α-Synuclein Oligomers Induce Glutamate Release from Astrocytes and Excessive Extrasynaptic NMDAR Activity in Neurons, Thus Contributing to Synapse Loss.
Trudler D, Sanz-Blasco S, Eisele YS, Ghatak S, Bodhinathan K, Akhtar MW, Lynch WP, Piña-Crespo JC, Talantova M, Kelly JW, Lipton SA. Trudler D, et al. J Neurosci. 2021 Mar 10;41(10):2264-2273. doi: 10.1523/JNEUROSCI.1871-20.2020. Epub 2021 Jan 22. J Neurosci. 2021. PMID: 33483428 Free PMC article. - Time-dependent effect of oligomeric amyloid-β (1-42)-induced hippocampal neurodegeneration in rat model of Alzheimer's disease.
Karthick C, Nithiyanandan S, Essa MM, Guillemin GJ, Jayachandran SK, Anusuyadevi M. Karthick C, et al. Neurol Res. 2019 Feb;41(2):139-150. doi: 10.1080/01616412.2018.1544745. Epub 2018 Nov 19. Neurol Res. 2019. PMID: 30453864 - Channel-mediated astrocytic glutamate release via Bestrophin-1 targets synaptic NMDARs.
Han KS, Woo J, Park H, Yoon BJ, Choi S, Lee CJ. Han KS, et al. Mol Brain. 2013 Jan 16;6:4. doi: 10.1186/1756-6606-6-4. Mol Brain. 2013. PMID: 23324492 Free PMC article. - Oligomeric Aβ-induced synaptic dysfunction in Alzheimer's disease.
Tu S, Okamoto S, Lipton SA, Xu H. Tu S, et al. Mol Neurodegener. 2014 Nov 14;9:48. doi: 10.1186/1750-1326-9-48. Mol Neurodegener. 2014. PMID: 25394486 Free PMC article. Review. - Reciprocal disruption of neuronal signaling and Aβ production mediated by extrasynaptic NMDA receptors: a downward spiral.
Rush T, Buisson A. Rush T, et al. Cell Tissue Res. 2014 May;356(2):279-86. doi: 10.1007/s00441-013-1789-1. Epub 2014 Feb 5. Cell Tissue Res. 2014. PMID: 24496511 Review.
Cited by
- Relationship between Zinc (Zn (2+) ) and Glutamate Receptors in the Processes Underlying Neurodegeneration.
Pochwat B, Nowak G, Szewczyk B. Pochwat B, et al. Neural Plast. 2015;2015:591563. doi: 10.1155/2015/591563. Epub 2015 May 27. Neural Plast. 2015. PMID: 26106488 Free PMC article. Review. - Physiological Roles of β-amyloid in Regulating Synaptic Function: Implications for AD Pathophysiology.
Cai W, Li L, Sang S, Pan X, Zhong C. Cai W, et al. Neurosci Bull. 2023 Aug;39(8):1289-1308. doi: 10.1007/s12264-022-00985-9. Epub 2022 Nov 28. Neurosci Bull. 2023. PMID: 36443453 Free PMC article. Review. - Astrocytes and Adenosine A2_A_ Receptors: Active Players in Alzheimer's Disease.
Lopes CR, Cunha RA, Agostinho P. Lopes CR, et al. Front Neurosci. 2021 May 13;15:666710. doi: 10.3389/fnins.2021.666710. eCollection 2021. Front Neurosci. 2021. PMID: 34054416 Free PMC article. Review. - Zika virus vertical transmission induces neuroinflammation and synapse impairment in brain cells derived from children born with Congenital Zika Syndrome.
Benazzato C, Lojudice F, Pöehlchen F, Leite PEC, Manucci AC, Van der Linden V, Jungmann P, Sogayar MC, Bruni-Cardoso A, Russo FB, Beltrão-Braga P. Benazzato C, et al. Sci Rep. 2024 Aug 3;14(1):18002. doi: 10.1038/s41598-024-65392-8. Sci Rep. 2024. PMID: 39097642 Free PMC article. - Going the Extra (Synaptic) Mile: Excitotoxicity as the Road Toward Neurodegenerative Diseases.
Armada-Moreira A, Gomes JI, Pina CC, Savchak OK, Gonçalves-Ribeiro J, Rei N, Pinto S, Morais TP, Martins RS, Ribeiro FF, Sebastião AM, Crunelli V, Vaz SH. Armada-Moreira A, et al. Front Cell Neurosci. 2020 Apr 24;14:90. doi: 10.3389/fncel.2020.00090. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32390802 Free PMC article. Review.
References
- Milnerwood AJ, et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington’s disease mice. Neuron. 2010;65(2):178–190. - PubMed
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5(5):405–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG010436/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- P30 NS076411/NS/NINDS NIH HHS/United States
- R01 NS050636/NS/NINDS NIH HHS/United States
- R01 AA020404/AA/NIAAA NIH HHS/United States
- T35 HL007491/HL/NHLBI NIH HHS/United States
- P01 HD029587/HD/NICHD NIH HHS/United States
- R01 AG010436/AG/NIA NIH HHS/United States
- P01 DA017259/DA/NIDA NIH HHS/United States
- P01 ES016738/ES/NIEHS NIH HHS/United States
- P01 HD29587/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials