Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization - PubMed (original) (raw)
Dysregulated left inferior parietal activity in schizophrenia and depression: functional connectivity and characterization
Veronika I Müller et al. Front Hum Neurosci. 2013.
Abstract
The inferior parietal cortex (IPC) is a heterogeneous region that is known to be involved in a multitude of diverse different tasks and processes, though its contribution to these often-complex functions is yet poorly understood. In a previous study we demonstrated that patients with depression failed to deactivate the left IPC during processing of congruent audiovisual information. We now found the same dysregulation (same region and condition) in schizophrenia. By using task-independent (resting state) and task-dependent meta-analytic connectivity modeling (MACM) analyses we aimed at characterizing this particular region with regard to its connectivity and function. Across both approaches, results revealed functional connectivity of the left inferior parietal seed region with bilateral IPC, precuneus and posterior cingulate cortex (PrC/PCC), medial orbitofrontal cortex (mOFC), left middle frontal (MFG) as well as inferior frontal (IFG) gyrus. Network-level functional characterization further revealed that on the one hand, all interconnected regions are part of a network involved in memory processes. On the other hand, sub-networks are formed when emotion, language, social cognition and reasoning processes are required. Thus, the IPC-region that is dysregulated in both depression and schizophrenia is functionally connected to a network of regions which, depending on task demands may form sub-networks. These results therefore indicate that dysregulation of left IPC in depression and schizophrenia might not only be connected to deficits in audiovisual integration, but is possibly also associated to impaired memory and deficits in emotion processing in these patient groups.
Keywords: depression; functional connectivity; inferior parietal cortex; resting-state; schizophrenia.
Figures
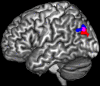
Figure 1
Significant interaction between incongruence × group in left inferior parietal cortex in depression (blue) and schizophrenia (red). The overlap of both activations served as seed area (purple) for functional connectivity calculation.
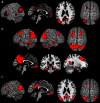
Figure 2
Results of the task-dependent (A), task-independent (B) analysis as well as the conjunction across both approaches (C). (A) Task-dependent functional connectivity of the seed region with bilateral parietal cortex extending into middle temporal and middle occipital gyri, with posterior cingulate cortex and precuneus, medial orbitofrontal cortex, left inferior frontal gyrus, left middle/superior frontal gyrus and left middle temporal gyrus. (B) Task-independent functional connectivity of the seed with bilateral parietal cortex extending into lateral occipital und temporal gyrus, precuneus and posterior cingulate cortex, as well as with bilateral cerebellum, hippocampus, parahippocampal gyrus, thalamus, fusiform gyrus, inferior and middle temporal gyrus, middle frontal gyrus, left inferior frontal gyrus, and medial orbitofrontal cortex. (C) Conjunction of task-dependent and task-independent connectivity reveals significant functional connectivity of the seed area with bilateral inferior parietal cortex, precuneus and posterior cingulate cortex, left middle and inferior frontal gyrus and medial orbitofrontal cortex.
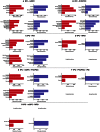
Figure 3
Significant behavioral domains and paradigm classes for the different sub-networks revealed by forward (red) and reverse inference (blue). Details can be found in the result section “Functional characterization of derived networks”.
Similar articles
- Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network.
Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, Jiang T, Zhou Y, Liu Z. Liu H, et al. Schizophr Bull. 2012 Mar;38(2):285-94. doi: 10.1093/schbul/sbq074. Epub 2010 Jun 30. Schizophr Bull. 2012. PMID: 20595202 Free PMC article. - Differential functional patterns of the human posterior cingulate cortex during activation and deactivation: a meta-analytic connectivity model.
Busler JN, Yanes JA, Bird RT, Reid MA, Robinson JL. Busler JN, et al. Exp Brain Res. 2019 Sep;237(9):2367-2385. doi: 10.1007/s00221-019-05595-y. Epub 2019 Jul 10. Exp Brain Res. 2019. PMID: 31292696 Free PMC article. - Functional connectivity of the orbitofrontal cortex, anterior cingulate cortex, and inferior frontal gyrus in humans.
Du J, Rolls ET, Cheng W, Li Y, Gong W, Qiu J, Feng J. Du J, et al. Cortex. 2020 Feb;123:185-199. doi: 10.1016/j.cortex.2019.10.012. Epub 2019 Nov 16. Cortex. 2020. PMID: 31869573 - Subspecialization in the human posterior medial cortex.
Bzdok D, Heeger A, Langner R, Laird AR, Fox PT, Palomero-Gallagher N, Vogt BA, Zilles K, Eickhoff SB. Bzdok D, et al. Neuroimage. 2015 Feb 1;106:55-71. doi: 10.1016/j.neuroimage.2014.11.009. Epub 2014 Nov 8. Neuroimage. 2015. PMID: 25462801 Free PMC article. - The structural and functional connectivity of the posterior cingulate cortex: comparison between deterministic and probabilistic tractography for the investigation of structure-function relationships.
Khalsa S, Mayhew SD, Chechlacz M, Bagary M, Bagshaw AP. Khalsa S, et al. Neuroimage. 2014 Nov 15;102 Pt 1:118-27. doi: 10.1016/j.neuroimage.2013.12.022. Epub 2013 Dec 21. Neuroimage. 2014. PMID: 24365673 Review.
Cited by
- Convergent functional effects of antidepressants in major depressive disorder: a neuroimaging meta-analysis.
Saberi A, Ebneabbasi A, Rahimi S, Sarebannejad S, Sen ZD, Graf H, Walter M, Sorg C, Camilleri JA, Laird AR, Fox PT, Valk SL, Eickhoff SB, Tahmasian M. Saberi A, et al. Mol Psychiatry. 2024 Oct 15. doi: 10.1038/s41380-024-02780-6. Online ahead of print. Mol Psychiatry. 2024. PMID: 39406999 - Illness-related variables and abnormalities of resting-state brain activity in schizophrenia.
Giuliani L, Pezzella P, Giordano GM, Fazio L, Mucci A, Perrottelli A, Blasi G, Amore M, Rocca P, Rossi A, Bertolino A, Galderisi S, Maj M. Giuliani L, et al. Front Psychiatry. 2024 Aug 6;15:1458624. doi: 10.3389/fpsyt.2024.1458624. eCollection 2024. Front Psychiatry. 2024. PMID: 39165501 Free PMC article. - Functional magnetic resonance imaging study of group independent components underpinning item responses to paranoid-depressive scale.
Stoyanov D, Paunova R, Dichev J, Kandilarova S, Khorev V, Kurkin S. Stoyanov D, et al. World J Clin Cases. 2023 Dec 26;11(36):8458-8474. doi: 10.12998/wjcc.v11.i36.8458. World J Clin Cases. 2023. PMID: 38188204 Free PMC article. - Analysis of EEG features and study of automatic classification in first-episode and drug-naïve patients with major depressive disorder.
Huang Y, Yi Y, Chen Q, Li H, Feng S, Zhou S, Zhang Z, Liu C, Li J, Lu Q, Zhang L, Han W, Wu F, Ning Y. Huang Y, et al. BMC Psychiatry. 2023 Nov 13;23(1):832. doi: 10.1186/s12888-023-05349-9. BMC Psychiatry. 2023. PMID: 37957613 Free PMC article. - Testosterone and the Amygdala's Functional Connectivity in Women and Men.
Kogler L, Müller VI, Moser E, Windischberger C, Gur RC, Habel U, Eickhoff SB, Derntl B. Kogler L, et al. J Clin Med. 2023 Oct 13;12(20):6501. doi: 10.3390/jcm12206501. J Clin Med. 2023. PMID: 37892639 Free PMC article.
References
- Amunts K., Kedo O., Kindler M., Pieperhoff P., Mohlberg H., Shah N. J., et al. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl.) 210, 343–352 10.1007/s00429-005-0025-5 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources