A natural O-ring optimizes the dispersal of fungal spores - PubMed (original) (raw)
A natural O-ring optimizes the dispersal of fungal spores
Joerg A Fritz et al. J R Soc Interface. 2013.
Abstract
The forcibly ejected spores of ascomycete fungi must penetrate several millimetres of nearly still air surrounding sporocarps to reach dispersive airflows, and escape is facilitated when a spore is launched with large velocity. To launch, the spores of thousands of species are ejected through an apical ring, a small elastic pore. The startling diversity of apical ring and spore shapes and dimensions make them favoured characters for both species descriptions and the subsequent inference of relationships among species. However, the physical constraints shaping this diversity and the adaptive benefits of specific morphologies are not understood. Here, we develop an elastohydrodynamic theory of the spore's ejection through the apical ring and demonstrate that to avoid enormous energy losses during spore ejection, the four principal morphological dimensions of spore and apical ring must cluster within a nonlinear one-dimensional subspace. We test this prediction using morphological data for 45 fungal species from two different classes and 18 families. Our sampling encompasses multiple loss and gain events and potentially independent origins of this spore ejection mechanism. Although the individual dimensions of the spore and apical ring are only weakly correlated with each other, they collapse into the predicted subspace with high accuracy. The launch velocity appears to be within 2 per cent of the optimum for over 90 per cent of all forcibly ejected species. Although the morphological diversity of apical rings and spores appears startlingly diverse, a simple principle can be used to organize it.
Keywords: elastohydrodynamics; fluid dynamics; fungi; morphological diversity; optimization; spore dispersal.
Figures
Figure 1.
The spore shooting apparatus. (a) Sporocarps on the stalk of a plant. (b) Flask-shaped sporocarp, containing five asci. (c) Upper part of an ascus with a mature spore close to the apical ring, which is still sealed. The length L and width W of the spore and the dimensions of the apical ring (ℓ, b, d) are indicated. (d) Spore moving at velocity U and deforming the apical ring at launch. A lubricating layer of fluid separates the spore from the ring. (e) The region where the spore first deforms the ring. Here, x measures the distance from the point where the spore starts to compress the ring; the gap thickness h varies with x and asymptotes to a constant value _h_0 at x > λ as described in the text. Dashed line denotes dry contact deformation.
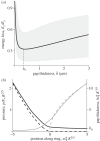
Figure 2.
Simulations of the elastohydrodynamics of apical ring deformation coupled with spore motion show the optimal thickness _h_∗ (equation (2.2)). (a) Total energy dissipated through friction and fluid loss El = _E_fluid + _E_friction, as a function of the average gap thickness  . We normalize El with the final spore kinetic energy
. We normalize El with the final spore kinetic energy  . Energy dissipation is minimized at the optimal gap thickness _h_∗, which is preserved as we vary the parameters of the model (G, C described in §4.1). Solid line and shading: results obtained for a realistic set of parameters (G = 0.05, C = 2.3, D = 0.79 μm1/2) and their expected variation, as described in the electronic supplementary material. (b) Normalized gap thickness (black) and pressure (grey) as a function of the distance from the point x = 0, where the spore first starts to compress the ring. The appropriate non-dimensionalization and numerical procedure are described in the electronic supplementary material. Dashed lines, solution of the dry contact problem; solid lines, solution of the full elastohydrodynamic problem.
. Energy dissipation is minimized at the optimal gap thickness _h_∗, which is preserved as we vary the parameters of the model (G, C described in §4.1). Solid line and shading: results obtained for a realistic set of parameters (G = 0.05, C = 2.3, D = 0.79 μm1/2) and their expected variation, as described in the electronic supplementary material. (b) Normalized gap thickness (black) and pressure (grey) as a function of the distance from the point x = 0, where the spore first starts to compress the ring. The appropriate non-dimensionalization and numerical procedure are described in the electronic supplementary material. Dashed lines, solution of the dry contact problem; solid lines, solution of the full elastohydrodynamic problem.
Figure 3.
Phylogenetic tree highlighting the 45 species used in this study (adapted from [20]). Classes and families with functional apical rings are in colour, those with non-functional rings are represented by grey dashed lines, and classes with other dispersal mechanism are shown in solid grey. (a) Cladogram of the entire ascomycete phylum, delineating classes. Clades with functional apical rings are the Leotiomycetes (blue), Sordariomycetes (red), Geoglossaceae (orange) and the Peltigeracea in the Lecanoromycetes (green). More detailed phylogeny of the Sordariomycetes (b) and of the Leotiomycetes (c) delineating families. The families of the species used in this study are highlighted. (d) Examples of apical ring geometries (not to scale) to illustrate morphological diversity (adapted from [–19]). Scale bars represents substitutions per site.
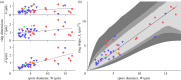
Figure 4.
Comparison between the theoretical prediction and real morphological data for 45 species represented in figure 3 by matching colours and symbols. (a) Correlation between the width of the spore W and individual dimensions of the ring. The _R_2 values of the correlations are 0.11, 0.10, 0.64 for ℓ, b, d, respectively. (b) The non-trivial combination of lengthscales predicted theoretically correlates well with spore width (_R_2 = 0.84). The line represents equation (2.7) with fitting parameter D = 0.79 μm1/2. Contour lines represent regions where spores attain 99% (light grey), 98% (grey) and 95% (dark grey) of the maximum ejecion velocity.
Figure 5.
Morphological analysis for 13 species with non-functional apical rings. Symbols represent classes (figure 3), species shown here are represented by dashed grey lines in figure 3. Contours as in figure 4_b_. Relaxation of the evolutionary constraint on the apical ring results in loss of optimality. No signature of the linear relation between W and _S_r, as predicted by equation (2.7) for functional apical rings, can be seen here (_R_2 = 0.076; _p_-value 0.32). The (inset) shows a positive correlation between the phylogentic distance from the last ancestor with a functional apical ring (in substitutions per site) and the loss in range compared with an optimal ring geometry for the same spore size (Z_opt−_Z)/_Z_opt. The distance from the last common ancestor is measured on species level phylogenies [31,32] using ancestral character reconstruction. The grey band corresponds to a 5% deviation from the optimum, which would contain all species with function apical rings. Note that three species are not shown in the inset, since their phylogenetic status in unclear.
Similar articles
- Explosively launched spores of ascomycete fungi have drag-minimizing shapes.
Roper M, Pepper RE, Brenner MP, Pringle A. Roper M, et al. Proc Natl Acad Sci U S A. 2008 Dec 30;105(52):20583-8. doi: 10.1073/pnas.0805017105. Epub 2008 Dec 22. Proc Natl Acad Sci U S A. 2008. PMID: 19104035 Free PMC article. - Dispersal of fungal spores on a cooperatively generated wind.
Roper M, Seminara A, Bandi MM, Cobb A, Dillard HR, Pringle A. Roper M, et al. Proc Natl Acad Sci U S A. 2010 Oct 12;107(41):17474-9. doi: 10.1073/pnas.1003577107. Epub 2010 Sep 28. Proc Natl Acad Sci U S A. 2010. PMID: 20880834 Free PMC article. - Asymmetric drop coalescence launches fungal ballistospores with directionality.
Liu F, Chavez RL, Patek SN, Pringle A, Feng JJ, Chen CH. Liu F, et al. J R Soc Interface. 2017 Jul;14(132):20170083. doi: 10.1098/rsif.2017.0083. J R Soc Interface. 2017. PMID: 28747394 Free PMC article. - Fungal cannons: explosive spore discharge in the Ascomycota.
Trail F. Trail F. FEMS Microbiol Lett. 2007 Nov;276(1):12-8. doi: 10.1111/j.1574-6968.2007.00900.x. Epub 2007 Sep 3. FEMS Microbiol Lett. 2007. PMID: 17784861 Review. - Role of Rain in the Spore Dispersal of Fungal Pathogens Associated with Grapevine Trunk Diseases.
Ji T, Altieri V, Salotti I, Li M, Rossi V. Ji T, et al. Plant Dis. 2024 Apr;108(4):1041-1052. doi: 10.1094/PDIS-03-23-0403-RE. Epub 2024 Apr 21. Plant Dis. 2024. PMID: 37822098 Review.
Cited by
- Apothecial Ancestry, Evolution, and Re-Evolution in Thelebolales (Leotiomycetes, Fungi).
Quijada L, Matočec N, Kušan I, Tanney JB, Johnston PR, Mešić A, Pfister DH. Quijada L, et al. Biology (Basel). 2022 Apr 11;11(4):583. doi: 10.3390/biology11040583. Biology (Basel). 2022. PMID: 35453781 Free PMC article. - Characterizing the gene-environment interaction underlying natural morphological variation in Neurospora crassa conidiophores using high-throughput phenomics and transcriptomics.
Krach EK, Skaro M, Wu Y, Arnold J. Krach EK, et al. G3 (Bethesda). 2022 Apr 4;12(4):jkac050. doi: 10.1093/g3journal/jkac050. G3 (Bethesda). 2022. PMID: 35293585 Free PMC article. - Timing of fungal spore release dictates survival during atmospheric transport.
Lagomarsino Oneto D, Golan J, Mazzino A, Pringle A, Seminara A. Lagomarsino Oneto D, et al. Proc Natl Acad Sci U S A. 2020 Mar 10;117(10):5134-5143. doi: 10.1073/pnas.1913752117. Epub 2020 Feb 25. Proc Natl Acad Sci U S A. 2020. PMID: 32098849 Free PMC article. - Fungal artillery of zombie flies: infectious spore dispersal using a soft water cannon.
de Ruiter J, Arnbjerg-Nielsen SF, Herren P, Høier F, De Fine Licht HH, Jensen KH. de Ruiter J, et al. J R Soc Interface. 2019 Oct 31;16(159):20190448. doi: 10.1098/rsif.2019.0448. Epub 2019 Oct 30. J R Soc Interface. 2019. PMID: 31662074 Free PMC article. - Shooting Mechanisms in Nature: A Systematic Review.
Sakes A, van der Wiel M, Henselmans PW, van Leeuwen JL, Dodou D, Breedveld P. Sakes A, et al. PLoS One. 2016 Jul 25;11(7):e0158277. doi: 10.1371/journal.pone.0158277. eCollection 2016. PLoS One. 2016. PMID: 27454125 Free PMC article. Review.
References
- Fisher MC, Henk DA, Briggs CJ, Brownstein JS, Madoff LC, McCraw SL, Gurr SJ. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484, 186–19410.1038/nature10947 (doi:10.1038/nature10947) - DOI - DOI - PMC - PubMed
- Pennisi E. 2010. Armed and dangerous. Science 327, 804–80510.1126/science.327.5967.804 (doi:10.1126/science.327.5967.804) - DOI - DOI - PubMed
- Trail F, Xu H, Loranger R, Gadoury D. 2002. Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia 94, 181–18910.2307/3761794 (doi:10.2307/3761794) - DOI - DOI - PubMed
- Trail F, Gaffoor I, Vogel S. 2005. Ejection mechanics and trajectory of the ascospores of Gibberella zeae (anamorph Fusarium graminearum). Fungal Genet. Biol. 42, 528–53310.1016/j.fgb.2005.03.008 (doi:10.1016/j.fgb.2005.03.008) - DOI - DOI - PubMed
- Linnaeus C. 1753. Species plantarum. Stockholm, Sweden: Impensis Laurentii Salvii
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources