A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome - PubMed (original) (raw)
Clinical Trial
A comparison of sex-specific immune signatures in Gulf War illness and chronic fatigue syndrome
Anne Liese Smylie et al. BMC Immunol. 2013.
Abstract
Background: Though potentially linked to the basic physiology of stress response we still have no clear understanding of Gulf War Illness (GWI), a debilitating condition presenting complex immune, endocrine and neurological symptoms. Here we compared male (n = 20) and female (n = 10) veterans with GWI separately against their healthy counterparts (n = 21 male, n = 9 female) as well as subjects with chronic fatigue syndrome/ myalgic encephalomyelitis (CFS/ME) (n = 12 male, n = 10 female).
Methods: Subjects were assessed using a Graded eXercise Test (GXT) with blood drawn prior to exercise, at peak effort (VO2 max) and 4-hours post exercise. Using chemiluminescent imaging we measured the concentrations of IL-1a, 1b, 2, 4, 5, 6, 8, 10, 12 (p70), 13, 15, 17 and 23, IFNγ, TNFα and TNFβ in plasma samples from each phase of exercise. Linear classification models were constructed using stepwise variable selection to identify cytokine co-expression patterns characteristic of each subject group.
Results: Classification accuracies in excess of 80% were obtained using between 2 and 5 cytokine markers. Common to both GWI and CFS, IL-10 and IL-23 expression contributed in an illness and time-dependent manner, accompanied in male subjects by NK and Th1 markers IL-12, IL-15, IL-2 and IFNγ. In female GWI and CFS subjects IL-10 was again identified as a delineator but this time in the context of IL-17 and Th2 markers IL-4 and IL-5. Exercise response also differed between sexes: male GWI subjects presented characteristic cytokine signatures at rest but not at peak effort whereas the opposite was true for female subjects.
Conclusions: Though individual markers varied, results collectively supported involvement of the IL-23/Th17/IL-17 axis in the delineation of GWI and CFS in a sex-specific way.
Figures
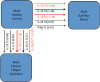
Figure 1
Gender differences in immune signatures among healthy controls. Immune cytokine expression of male and female healthy controls was compared using a step-wise method across all time points (rest, peak activity, and recovery). Red arrows indicate a negative contribution of a particular cytokine in female healthy controls compared to males and vice versa for black arrows. Arrow line thickness is proportional to the magnitude of the standardized canonical coefficients. Changes in IL-23 were a dominant factor at all time points, in particular when cast against concurrent levels of IL-2 and IL-5 expression under challenge.
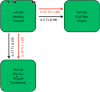
Figure 2
Cytokine signatures separating illness groups in male subjects. Immune cytokine expression patterns separating of male GWI, CFS and healthy control subjects identified using a step-wise selection method across all time points (rest, peak activity, and recovery). Red arrows indicate a negative contribution, vice versa for black arrows. Arrow line thickness is proportional to the magnitude of standardized canonical coefficients. NK cell promoters (IL-12 or 15) are observed in both illnesses, as are IL-10 and 23. Stronger IL-2 expression was characteristic of CFS while stronger IL-13 contribution was indicative of GWI (Table 2 and Additional file 2: Table S5).
Figure 3
Cytokine signatures separating illness groups in female subjects. Immune cytokine expression patterns separating of female GWI, CFS and healthy control subjects identified using a step-wise selection method across all time points (rest, peak activity, and recovery). Red arrows indicate a negative contribution, vice versa for black arrows. Arrow line thickness is proportional to the magnitude of standardized canonical coefficients. While decreased IL-23 expression in the context of increased IL-5 levels provided strong separation of GWI from healthy controls the signal for CFS was much weaker and required relaxation of the constraints on cross-correlation between cytokines.
Similar articles
- Altered immune pathway activity under exercise challenge in Gulf War Illness: an exploratory analysis.
Broderick G, Ben-Hamo R, Vashishtha S, Efroni S, Nathanson L, Barnes Z, Fletcher MA, Klimas N. Broderick G, et al. Brain Behav Immun. 2013 Feb;28:159-69. doi: 10.1016/j.bbi.2012.11.007. Epub 2012 Nov 29. Brain Behav Immun. 2013. PMID: 23201588 - Cytokine expression provides clues to the pathophysiology of Gulf War illness and myalgic encephalomyelitis.
Khaiboullina SF, DeMeirleir KL, Rawat S, Berk GS, Gaynor-Berk RS, Mijatovic T, Blatt N, Rizvanov AA, Young SG, Lombardi VC. Khaiboullina SF, et al. Cytokine. 2015 Mar;72(1):1-8. doi: 10.1016/j.cyto.2014.11.019. Epub 2014 Dec 13. Cytokine. 2015. PMID: 25514671 Free PMC article. - Exploring the Diagnostic Potential of Immune Biomarker Co-expression in Gulf War Illness.
Broderick G, Fletcher MA, Gallagher M, Barnes Z, Vernon SD, Klimas NG. Broderick G, et al. Methods Mol Biol. 2018;1781:101-120. doi: 10.1007/978-1-4939-7828-1_7. Methods Mol Biol. 2018. PMID: 29705845 - Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment.
White RF, Steele L, O'Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R. White RF, et al. Cortex. 2016 Jan;74:449-75. doi: 10.1016/j.cortex.2015.08.022. Epub 2015 Sep 25. Cortex. 2016. PMID: 26493934 Free PMC article. Review.
Cited by
- Blood Biomarkers of Chronic Inflammation in Gulf War Illness.
Johnson GJ, Slater BC, Leis LA, Rector TS, Bach RR. Johnson GJ, et al. PLoS One. 2016 Jun 28;11(6):e0157855. doi: 10.1371/journal.pone.0157855. eCollection 2016. PLoS One. 2016. PMID: 27352030 Free PMC article. - The impact of post-traumatic stress on quality of life and fatigue in women with Gulf War Illness.
Shastry N, Sultana E, Jeffrey M, Collado F, Kibler J, DeLucia C, Fletcher MA, Klimas N, Craddock TJA. Shastry N, et al. BMC Psychol. 2022 Feb 25;10(1):42. doi: 10.1186/s40359-022-00752-5. BMC Psychol. 2022. PMID: 35216624 Free PMC article. - Exercise benefits the cardiac, autonomic and inflammatory responses to organophosphate toxicity.
Freire Machi J, Schmidt R, Salgueiro LM, Fernandes Stoyell-Conti F, de Andrade Barboza C, Hernandez DR, Morris M. Freire Machi J, et al. Toxicol Rep. 2019 Jun 26;6:666-673. doi: 10.1016/j.toxrep.2019.06.015. eCollection 2019. Toxicol Rep. 2019. PMID: 31673494 Free PMC article. - Exercise Pathophysiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Postacute Sequelae of SARS-CoV-2: More in Common Than Not?
Joseph P, Singh I, Oliveira R, Capone CA, Mullen MP, Cook DB, Stovall MC, Squires J, Madsen K, Waxman AB, Systrom DM. Joseph P, et al. Chest. 2023 Sep;164(3):717-726. doi: 10.1016/j.chest.2023.03.049. Epub 2023 Apr 11. Chest. 2023. PMID: 37054777 Free PMC article. Review. - Tracking post-infectious fatigue in clinic using routine Lab tests.
Harvey JM, Broderick G, Bowie A, Barnes ZM, Katz BZ, O'Gorman MRG, Vernon SD, Fletcher MA, Klimas NG, Taylor R. Harvey JM, et al. BMC Pediatr. 2016 Apr 26;16:54. doi: 10.1186/s12887-016-0596-8. BMC Pediatr. 2016. PMID: 27118537 Free PMC article. Clinical Trial.
References
- Eisen SA, Kang HK, Murphy FM, Blanchard MS, Reda DJ, Henderson WG, Toomey R, Jackson LW, Alpern R, Parks BJ, Klimas N, Hall C, Pak HS, Hunter J, Karlinsky J, Battistone MJ, Lyons MJ. Gulf War Study Participating Investigators: Gulf War veterans’ health: medical evaluation of a US cohort. Ann Int Med. 2005;142:881–890. doi: 10.7326/0003-4819-142-11-200506070-00005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical