Tired and apprehensive: anxiety amplifies the impact of sleep loss on aversive brain anticipation - PubMed (original) (raw)
Tired and apprehensive: anxiety amplifies the impact of sleep loss on aversive brain anticipation
Andrea N Goldstein et al. J Neurosci. 2013.
Abstract
Anticipation is an adaptive process, aiding preparatory responses to potentially threatening events. However, excessive anticipatory responding and associated hyper-reactivity in the amygdala and insula are integral to anxiety disorders. Despite the co-occurrence of sleep disruption and anxiety disorders, the impact of sleep loss on affective anticipatory brain mechanisms, and the interaction with anxiety, remains unknown. Here, we demonstrate that sleep loss amplifies preemptive responding in the amygdala and anterior insula during affective anticipation in humans, especially for cues with high predictive certainty. Furthermore, trait anxiety significantly determined the degree of such neural vulnerability to sleep loss: individuals with highest trait anxiety showed the greatest increase in anticipatory insula activity when sleep deprived. Together, these data support a neuropathological model in which sleep disruption may contribute to the maintenance and/or exacerbation of anxiety through its impact on anticipatory brain function. They further raise the therapeutic possibility that targeted sleep restoration in anxiety may ameliorate excessive anticipatory responding and associated clinical symptomatology.
Figures
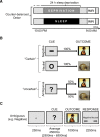
Figure 1.
Study design. A, Time course of experiment for both the sleep-deprived and sleep-rested conditions. B, fMRI anticipation reactivity task trial types. Each trial consisted of an anticipatory cue followed by either an emotional negative or neutral picture stimulus. For negative trials, the anticipation cue was a red “−” symbol that was always followed by a negative picture. For the neutral trials, the anticipation cue was a yellow “O” symbol that was always followed by a neutral picture. For ambiguous trials, the anticipation cue was a white “?” symbol, which was followed by either a negative or a neutral picture at exactly a 50:50 ratio. C, An example anticipation reactivity task trial with timing. Each anticipation trial lasted an average of 8.75 s. Following a fixation screen, the trial proceeded with (1) the cued anticipation phase, in which one of the three warning cues were presented using a variable jittered time duration for optimal signal estimation (maximum, 5.6 s; minimum, 2.5 s; mean, 4.5 s) (Dale, 1999); (2) the stimulus reactivity phase, in which the emotionally negative or neutral image was presented (1 s); and (3) the response period, where subjects made a categorical emotionality judgment using a button-press rating (negative/neutral; 2.5 s), also confirming wakefulness. Pseudorandomly interspersed between these anticipation trials were null trial events (data not shown), where a fixation point was displayed on the screen for a jittered duration (maximum, 5 s; minimum, 1.5 s; mean, 2.5 s), serving as both a baseline condition as well as modulating intertrial interval variability for optimal modeling of trial events.
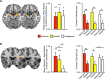
Figure 2.
A, Brain maps displaying ROI mask (left brain image) and the ANOVA main effect of condition (sleep deprivation, sleep rested; right brain image) from the voxelwise ROI analysis in bilateral amygdala across all three cue types (negative, neutral, and ambiguous) [peak MNI coordinates (x, y, z); left: −18, −6, −16; right: 24, −2, −16]. Right side bar graphs illustrate the difference in average parameter estimates across the 6 mm ROI spheres between the sleep-deprivation and sleep-rested conditions, and average signal for each condition, respectively. B, Brain maps displaying ROI mask (left brain image) and the ANOVA condition–cue type (negative, neutral, and ambiguous) interaction results from the voxelwise ROI analysis in the right anterior insula for the three cue types [peak MNI coordinates (x, y, z); 32, 20, 14], together with equivalent bar graphs as in A. No significant main effect of condition or cue type interaction was observed in the left anterior insula at FWE p < 0.05. Error bars represent SEM. Within-region statistics: *p < 0.05, **p < 0.01.
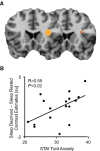
Figure 3.
A, Brain maps displaying anterior insula ROI mask (left brain image) and the voxelwise ROI regression between STAI trait anxiety and the relative sleep deprivation induced increase in anticipatory response in anterior insula for all cues [peak MNI coordinates (x, y, z); 34, 18, 18; T = 4.04, p < 0.001]. Regression thresholded at p < 0.05 FWE-corrected for multiple comparisons. B, Scatterplot displaying this same relationship using the average parameter estimates across the 6 mm ROI anterior insula ROI mask (left brain image).
Similar articles
- Feeling anxious: anticipatory amygdalo-insular response predicts the feeling of anxious anticipation.
Carlson JM, Greenberg T, Rubin D, Mujica-Parodi LR. Carlson JM, et al. Soc Cogn Affect Neurosci. 2011 Jan;6(1):74-81. doi: 10.1093/scan/nsq017. Epub 2010 Mar 5. Soc Cogn Affect Neurosci. 2011. PMID: 20207692 Free PMC article. - Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli.
Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Simmons AN, et al. Hum Brain Mapp. 2011 Nov;32(11):1836-46. doi: 10.1002/hbm.21154. Epub 2010 Dec 22. Hum Brain Mapp. 2011. PMID: 21181800 Free PMC article. - Dissecting the anticipation of aversion reveals dissociable neural networks.
Grupe DW, Oathes DJ, Nitschke JB. Grupe DW, et al. Cereb Cortex. 2013 Aug;23(8):1874-83. doi: 10.1093/cercor/bhs175. Epub 2012 Jul 4. Cereb Cortex. 2013. PMID: 22763169 Free PMC article. - Anticipatory affect: neural correlates and consequences for choice.
Knutson B, Greer SM. Knutson B, et al. Philos Trans R Soc Lond B Biol Sci. 2008 Dec 12;363(1511):3771-86. doi: 10.1098/rstb.2008.0155. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 18829428 Free PMC article. Review. - Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective.
Grupe DW, Nitschke JB. Grupe DW, et al. Nat Rev Neurosci. 2013 Jul;14(7):488-501. doi: 10.1038/nrn3524. Nat Rev Neurosci. 2013. PMID: 23783199 Free PMC article. Review.
Cited by
- (Re)Conceptualizing Sleep Among Children with Anxiety Disorders: Where to Next?
Alfano CA. Alfano CA. Clin Child Fam Psychol Rev. 2018 Dec;21(4):482-499. doi: 10.1007/s10567-018-0267-4. Clin Child Fam Psychol Rev. 2018. PMID: 30136070 Review. - The Impact of Problematic Smartphone Use on Sleep Quality Among Chinese Young Adults: Investigating Anxiety and Depression as Mediators in a Three-Wave Longitudinal Study.
Wu R, Niu Q, Wang Y, Dawa Y, Guang Z, Song D, Xue B, Lu C, Wang S. Wu R, et al. Psychol Res Behav Manag. 2024 Apr 29;17:1775-1786. doi: 10.2147/PRBM.S455955. eCollection 2024. Psychol Res Behav Manag. 2024. PMID: 38707963 Free PMC article. - The effect of physical activity on anxiety through sleep quality among Chinese high school students: evidence from cross-sectional study and longitudinal study.
Chen X, Yang Y, Zhong C, Zeng X, Qiu X, Zhou X, Liu C, Tian Z, Liu B, Yin R. Chen X, et al. BMC Psychiatry. 2025 May 16;25(1):495. doi: 10.1186/s12888-025-06909-x. BMC Psychiatry. 2025. PMID: 40380339 Free PMC article. - Affective neural responses modulated by serotonin transporter genotype in clinical anxiety and depression.
Oathes DJ, Hilt LM, Nitschke JB. Oathes DJ, et al. PLoS One. 2015 Feb 12;10(2):e0115820. doi: 10.1371/journal.pone.0115820. eCollection 2015. PLoS One. 2015. PMID: 25675343 Free PMC article. - Effect of trait anxiety on prefrontal control mechanisms during emotional conflict.
Comte M, Cancel A, Coull JT, Schön D, Reynaud E, Boukezzi S, Rousseau PF, Robert G, Khalfa S, Guedj E, Blin O, Weinberger DR, Fakra E. Comte M, et al. Hum Brain Mapp. 2015 Jun;36(6):2207-14. doi: 10.1002/hbm.22765. Epub 2015 Feb 9. Hum Brain Mapp. 2015. PMID: 25664956 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- R01 MH093537/MH/NIMH NIH HHS/United States
- F31-MH094075/MH/NIMH NIH HHS/United States
- R21-DA031939/DA/NIDA NIH HHS/United States
- R21 DA031939/DA/NIDA NIH HHS/United States
- F31 MH094075/MH/NIMH NIH HHS/United States
- R01-MH093537/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical