Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up - PubMed (original) (raw)
Randomized Controlled Trial
. 2013 Sep 1;188(5):567-76.
doi: 10.1164/rccm.201304-0651OC.
Collaborators, Affiliations
- PMID: 23805899
- PMCID: PMC3827703
- DOI: 10.1164/rccm.201304-0651OC
Randomized Controlled Trial
Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up
Dale M Needham et al. Am J Respir Crit Care Med. 2013.
Abstract
Rationale: We hypothesized that providing patients with acute lung injury two different protein/calorie nutritional strategies in the intensive care unit may affect longer-term physical and cognitive performance.
Objectives: To assess physical and cognitive performance 6 and 12 months after acute lung injury, and to evaluate the effect of trophic versus full enteral feeding, provided for the first 6 days of mechanical ventilation, on 6-minute-walk distance, cognitive impairment, and secondary outcomes.
Methods: A prospective, longitudinal ancillary study of the ARDS Network EDEN trial evaluating 174 consecutive survivors from 5 of 12 centers. Blinded assessments of patients' arm anthropometrics, strength, pulmonary function, 6-minute-walk distance, and cognitive status (executive function, language, memory, verbal reasoning/concept formation, and attention) were performed.
Measurements and main results: At 6 and 12 months, respectively, the mean (SD) percent predicted for 6-minute-walk distance was 64% (22%) and 66% (25%) (P = 0.011 for difference between assessments), and 36 and 25% of survivors had cognitive impairment (P = 0.001). Patients performed below predicted values for secondary physical tests with small improvement from 6 to 12 months. There was no significant effect of initial trophic versus full feeding for the first 6 days after randomization on survivors' percent predicted for 6-minute-walk distance, cognitive impairment status, and all secondary outcomes.
Conclusions: EDEN trial survivors performed below predicted values for physical and cognitive performance at 6 and 12 months, with some improvement over time. Initial trophic versus full enteral feeding for the first 6 days after randomization did not affect physical and cognitive performance.
Figures
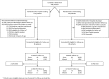
Figure 1.
Enrollment and follow-up.
Figure 2.
Effect size of treatment intervention at 12 months. The treatment effect, presented as an effect size, with 95% confidence interval, for the primary outcomes (6-min-walk test % predicted, and cognitive impairment) and all secondary outcomes. Effect size was calculated as the treatment effect (Table 3, difference in means or proportions) divided by the pooled SD from the initial trophic and full feeding groups (77, 78).
Comment in
- Feast or famine in the intensive care unit: does it really matter?
Hart N, Barreiro E. Hart N, et al. Am J Respir Crit Care Med. 2013 Sep 1;188(5):523-5. doi: 10.1164/rccm.201306-1162ED. Am J Respir Crit Care Med. 2013. PMID: 23992585 No abstract available.
References
- Griffiths RD. Nutrition and survival in intensive care. Med Klin Intensivemed Notfmed. 1998;35:3–9.
- Griffiths RD. Nutrition for critically ill patients: how much is enough? JAMA. 2012;307:845–846. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL096504/HL/NHLBI NIH HHS/United States
- R01HL091760-02S1/HL/NHLBI NIH HHS/United States
- UL1 TR000424/TR/NCATS NIH HHS/United States
- R01 HL091760/HL/NHLBI NIH HHS/United States
- R34 HL105869/HL/NHLBI NIH HHS/United States
- UL1 TR 000424-06/TR/NCATS NIH HHS/United States
- R01HL091760/HL/NHLBI NIH HHS/United States
- R01HL096504/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical