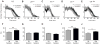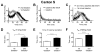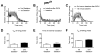Circadian genes differentially affect tolerance to ethanol in Drosophila - PubMed (original) (raw)
Circadian genes differentially affect tolerance to ethanol in Drosophila
Jascha B Pohl et al. Alcohol Clin Exp Res. 2013 Nov.
Abstract
Background: There is a strong relationship between circadian rhythms and ethanol (EtOH) responses. EtOH consumption has been shown to disrupt physiological and behavioral circadian rhythms in mammals (Alcohol Clin Exp Res 2005b, 29, 1550). The Drosophila central circadian pacemaker is composed of proteins encoded by the per, tim, cyc, and Clk genes. Using Drosophila mutant analysis, we asked whether these central components of the circadian clock make the equivalent contribution toward EtOH tolerance and whether rhythmicity itself is necessary for tolerance.
Methods: We tested flies carrying mutations in core clock genes for the capacity to acquire EtOH tolerance. Tolerance was assayed by comparing the sedation curves of populations during their first and second sedation. Animals that had acquired tolerance sedated more slowly. Movement was also monitored as the flies breathe the EtOH vapor to determine if other facets of the EtOH response were affected by the mutations. Gas chromatography was used to measure internal EtOH concentration. Constant light was used to nongenetically destabilize the PER and TIM proteins.
Results: A group of circadian mutations, all of which eliminate circadian rhythms, do not disrupt tolerance identically. Mutations in per, tim, and cyc completely block tolerance. However, a mutation in Clk does not interfere with tolerance. Constant light also disrupts the capacity to acquire tolerance. These lines did not differ in EtOH absorption.
Conclusions: Mutations affecting different parts of the intracellular circadian clock can block the capacity to acquire rapid EtOH tolerance. However, the role of circadian genes in EtOH tolerance is independent of their role in producing circadian rhythmicity. The interference in the capacity to acquire EtOH tolerance by some circadian mutations is not merely a downstream effect of a nonfunctional circadian clock; instead, these circadian genes play an independent role in EtOH tolerance.
Keywords: Alcohol Tolerance; Circadian Rhythm; Drosophila; Mutation.
Copyright © 2013 by the Research Society on Alcoholism.
Figures
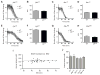
Figure 1. Circadian mutants do not affect sensitivity to ethanol
Knockdown curves are used to compare the ethanol sensitivity of the wild type and the various backcrossed circadian mutant alleles. In each plot, the sensitivity of the wild-type Canton S line is compared with a circadian mutant that has been backcrossed into the wild-type background. Shown are A) _per_01, C) _tim_01, E) _cyc_01, and G) _Clk_JRK. Bar graphs compare the time required for 50% to be knocked down (K50) by the ethanol vapor from a 35% ethanol solution for B) _per_01, D) _tim_01, F) _cyc_01, and H) _Clk_JRK. None of the comparisons were significantly different. Statistical significance was determined using Student's t test with a cutoff of p<0.05. n=4 vials for _per_01 test, n=6 vials for all others. Each vial contains 10 animals. **I)** The K50 occurs at a specific internal ethanol concentration. Thirty-nine ethanol sedations (10 flies per sedation) were performed on different days or with different ethanol concentrations (35% or 40% ethanol was used). This produced wide variation in the K50 value (29 minutes to 71 minutes). When half of the flies were sedated (K50) the flies were sacrificed and the internal ethanol concentration measured (111 mM +/−2.3, N=39). The slope of the best fit line does not differ significantly from zero (r2<0.002, P>0.8). J) The CS (wild type) and mutant _per_01, _tim_01, _cyc_01, and _Clk_JRK stocks do not differ from one another in their internal ethanol hemolymph concentration after vapor treatment. Internal ethanol content was determined by gas chromatography after 30 minutes of exposure to the vapor from a 35% ethanol solution. Ethanol treatment is identical to panes A–H except that all stocks were treated at the same time. The lack of statistical significance was determined using one way Anova with the Dunnett's Multiple Comparison Test. Error bars are standard error of the mean, n= 6 vials. Each vial contains 10 animals.
Figure 2. Some circadian mutants disrupt tolerance to ethanol
Shown are knockdown curves that compare flies receiving their first sedation to flies receiving their second sedation for A) CS, B) per_01,_ C) _tim_01, D) _cyc_01, and E) _Clk_JRK. The corresponding bar graph for K50 is shown for F) CS, G) _per_01, H) _tim_01, I) _cyc_01, and J) _Clk_JRK. Statistical significance was determined using Student's t test (* p<0.05, **p<0.01; n=4 vials for _per_01 test, n=6 vials for all others; each vial contains 10 animals).
Figure 3. A movement assay shows distinct attributes of tolerance
A) An activity plot of wild-type Canton S flies that compares the number of flies moving after receiving their first or second dose of ethanol. B) A baseline movement activity plot of the wild type flies placed in the test chamber without ethanol vapor. The baseline flies were mock-sedated 24 h before being placed in the test chamber. The baseline after ETOH flies were sedated with ethanol 24 h before being placed in a test chamber without ethanol. C) The ethanol component of the response curve was isolated by subtracting the baseline data (panel B) from the raw ethanol response data (panel A). The 1st minus baseline curve is produced by subtracting the baseline curve (panel B) from the 1st sedation curve (panel A). The 2nd minus baseline after ethanol curve was produced by subtracting the baseline after ETOH (panel B) from the 2nd sedation data (panel A). Error bars are standard error of the mean (n=6). The dotted white line is a nonlinear best-fit of single Gaussian curve to the data. Bar graphs were derived from the data in panel C and depict D) the T50 of the rising phase, which is the time at which the rises to 50% maximum amplitude, E) the time of maximal movement activity (Tmax), and F) the T50 of the falling phase, which is the time at which the curve decays to 50% maximum amplitude. The wild type shows evidence that it has acquired ethanol tolerance in all three of these parameters. Statistical significance was determined using a two-tailed Student's t test (**p=0.0016; ***p<0.001; n=6 vials; each vial contains 10 animals).
Figure 4. A movement assay shows that flies carrying the _per_01 mutation do not acquire any attributes of rapid tolerance
A) An activity plot that compares the number of flies moving for _per_01 flies that are receiving their first or second dose of ethanol. B) A baseline movement activity plot of _per_01 flies was generated as described in figure 2. C) The ethanol component of the response curve was isolated as described in figure 2. The _per_01 mutants do not show evidence of rapid ethanol tolerance in the the T50 of the rising phase of the activity curve (D), the Tmax of the activity curve (E), or in the T50 of the falling phase of the activity curve (F). Statistical significance was determined using a two-tailed Student's t test (n=4 vials; each vial contains 10 animals).
Figure 5. A movement assay shows that flies carrying the _tim_01 mutation do not acquire any attributes of rapid tolerance
A) An activity plot that compares the number of flies moving for _tim_01 flies receiving their first or second dose of ethanol. B) A baseline movement activity plot of _tim_01 flies was generated as described in figure 2. C) The ethanol component of the response curve was isolated as described in figure 2. The _tim_01 mutants do not show evidence of rapid ethanol tolerance in the the T50 of the rising phase of the activity curve (D), the Tmax of the activity curve (E), or in the T50 of the falling phase of the activity curve (F). Statistical significance was determined using a two-tailed Student's t test (n=6 vials; each vial contains 10 animals).
Figure 6. A movement assay shows that the _cyc_01 mutation disrupts some but not all aspects of the tolerance response
A) An activity plot that compares the number of flies moving for _cyc_01 flies receiving their first or second dose of ethanol. B) A baseline movement activity plot of _cyc_01 flies was generated as described in figure 2. C) The ethanol component of the response curve was isolated as described in figure 2. The _cyc_01 mutants do not show evidence of rapid ethanol tolerance in the the T50 of the rising phase of the activity curve (D), the Tmax of the activity curve (E), or in the T50 of the falling phase of the activity curve (F). Statistical significance was determined using a two-tailed Student's t test (n=6 vials; each vial contains 10 animals).
Figure 7. A movement assay shows that the _Clk_JRK mutation does not disrupt tolerance
A) An activity plot that compares the number of flies moving for _Clk_JRK flies receiving their first or second dose of ethanol. B) A baseline movement activity plot of _Clk_JRK flies was generated as described in figure 2. C) The ethanol component of the response curve was isolated as described in figure 2. The _Clk_JRK mutants show evidence that they have acquired rapid ethanol tolerance in the T50 of the rising phase of the activity curve (D), the Tmax of the activity curve (E), or in the T50 of the falling phase of the activity curve (F). Statistical significance was determined using a two-tailed Student's t test (**p<0.005; n=6 vials; each vial contains 10 animals).
Figure 8. Constant light eliminates ethanol tolerance in wild-type flies
Knockdown curves are shown comparing flies receiving their first sedation to flies receiving their second sedation for A) flies maintained in a 12:12 light:dark cycle and C) flies maintained in constant light. Corresponding bar graphs depicting K50 comparing flies receiving their first and second treatment are shown for B) Light:Dark flies and D) Constant light flies. Under 12:12 light dark conditions the rhythm index measured over three days was 0.139+/− 0.011 (n=24) while under constant light conditions the rhythm index dropped to 0.041 +/− 0.0009 (n=23). Statistical significance was determined using Student's t test (**p<0.01; n=6 vials; each vial contains 10 animals).
Similar articles
- F654A and K558Q Mutations in NMDA Receptor 1 Affect Ethanol-Induced Behaviors in Drosophila.
Troutwine B, Park A, Velez-Hernandez ME, Lew L, Mihic SJ, Atkinson NS. Troutwine B, et al. Alcohol Clin Exp Res. 2019 Dec;43(12):2480-2493. doi: 10.1111/acer.14215. Epub 2019 Nov 1. Alcohol Clin Exp Res. 2019. PMID: 31593608 Free PMC article. - The mammalian circadian clock in the suprachiasmatic nucleus exhibits rapid tolerance to ethanol in vivo and in vitro.
Lindsay JH, Glass JD, Amicarelli M, Prosser RA. Lindsay JH, et al. Alcohol Clin Exp Res. 2014 Mar;38(3):760-9. doi: 10.1111/acer.12303. Epub 2014 Feb 11. Alcohol Clin Exp Res. 2014. PMID: 24512529 - An RNAi Screen To Identify Protein Phosphatases That Function Within the Drosophila Circadian Clock.
Agrawal P, Hardin PE. Agrawal P, et al. G3 (Bethesda). 2016 Dec 7;6(12):4227-4238. doi: 10.1534/g3.116.035345. G3 (Bethesda). 2016. PMID: 27784754 Free PMC article. - Genetics and molecular biology of rhythms in Drosophila and other insects.
Hall JC. Hall JC. Adv Genet. 2003;48:1-280. doi: 10.1016/s0065-2660(03)48000-0. Adv Genet. 2003. PMID: 12593455 Review. - A Brief Overview of Ethanol Tolerance and Its Potential Association with Circadian Rhythm in Drosophila.
Peterson SK, Ahmad ST. Peterson SK, et al. Int J Mol Sci. 2024 Nov 24;25(23):12605. doi: 10.3390/ijms252312605. Int J Mol Sci. 2024. PMID: 39684317 Free PMC article. Review.
Cited by
- Repeated ethanol intoxications of Drosophila melanogaster adults increases the resistance to ethanol of their progeny.
Bonilla M, McPherson M, Coreas J, Boulos M, Chavol P, Alrabadi RI, Loza-Coll M. Bonilla M, et al. Alcohol Clin Exp Res. 2021 Jul;45(7):1370-1382. doi: 10.1111/acer.14647. Epub 2021 Jul 5. Alcohol Clin Exp Res. 2021. PMID: 34120365 Free PMC article. - Alcohol tolerance encoding in sleep regulatory circadian neurons in Drosophila.
Lange AP, Wolf FW. Lange AP, et al. bioRxiv [Preprint]. 2023 Feb 2:2023.01.30.526363. doi: 10.1101/2023.01.30.526363. bioRxiv. 2023. PMID: 36778487 Free PMC article. Updated. Preprint. - Mechanisms Underlying the Risk to Develop Drug Addiction, Insights From Studies in Drosophila melanogaster.
Ryvkin J, Bentzur A, Zer-Krispil S, Shohat-Ophir G. Ryvkin J, et al. Front Physiol. 2018 Apr 24;9:327. doi: 10.3389/fphys.2018.00327. eCollection 2018. Front Physiol. 2018. PMID: 29740329 Free PMC article. Review. - Mutations in the circadian gene period alter behavioral and biochemical responses to ethanol in Drosophila.
Liao J, Seggio JA, Ahmad ST. Liao J, et al. Behav Brain Res. 2016 Apr 1;302:213-9. doi: 10.1016/j.bbr.2016.01.041. Epub 2016 Jan 20. Behav Brain Res. 2016. PMID: 26802726 Free PMC article. - The FlyBar: administering alcohol to flies.
van der Linde K, Fumagalli E, Roman G, Lyons LC. van der Linde K, et al. J Vis Exp. 2014 May 18;(87):50442. doi: 10.3791/50442. J Vis Exp. 2014. PMID: 24895004 Free PMC article.
References
- Andretic R, Chaney S, Hirsh J. Requirement of circadian genes for cocaine sensitization in Drosophila. Science. 1999;285:1066–1068. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases