Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome - PubMed (original) (raw)
Randomized Controlled Trial
. 2013 Oct;24(10):1689-97.
doi: 10.1681/ASN.2012121200. Epub 2013 Jun 27.
Collaborators, Affiliations
- PMID: 23813218
- PMCID: PMC3785276
- DOI: 10.1681/ASN.2012121200
Randomized Controlled Trial
Mycophenolate mofetil versus cyclosporin A in children with frequently relapsing nephrotic syndrome
Jutta Gellermann et al. J Am Soc Nephrol. 2013 Oct.
Abstract
The severe side effects of long-term corticosteroid or cyclosporin A (CsA) therapy complicate the treatment of children with frequently relapsing steroid-sensitive nephrotic syndrome (FR-SSNS). We conducted a randomized, multicenter, open-label, crossover study comparing the efficacy and safety of a 1-year treatment with mycophenolate mofetil (MMF; target plasma mycophenolic acid trough level of 1.5-2.5 µg/ml) or CsA (target trough level of 80-100 ng/ml) in 60 pediatric patients with FR-SSNS. We assessed the frequency of relapse as the primary endpoint and evaluated pharmacokinetic profiles (area under the curve [AUC]) after 3 and 6 months of treatment. More relapses per patient per year occurred with MMF than with CsA during the first year (P=0.03), but not during the second year (P=0.14). No relapses occurred in 85% of patients during CsA therapy and in 64% of patients during MMF therapy (P=0.06). However, the time without relapse was significantly longer with CsA than with MMF during the first year (P<0.05), but not during the second year (P=0.36). In post hoc analysis, patients with low mycophenolic acid exposure (AUC <50 µg⋅h/ml) experienced 1.4 relapses per year compared with 0.27 relapses per year in those with high exposure (AUC>50 µg⋅h/ml; P<0.05). There were no significant differences between groups with respect to BP, growth, lipid levels, or adverse events. However, cystatin clearance, estimated GFR, and hemoglobin levels increased significantly with MMF compared with CsA. These results indicate that MMF is inferior to CsA in preventing relapses in pediatric patients with FR-SSNS, but may be a less nephrotoxic treatment option.
Figures
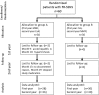
Figure 1.
Diagram of patient flow through the clinical trial.
Figure 2.
Efficacy of CsA and MMF in preventing relapses in FR-SSNS patients. Kaplan–Meier survival analysis: Time without relapse (cumulative sustained remission) during treatment with CsA or MMF. (A) In the first treatment year (P<0.05, long-rank test). (B) In the second treatment year (_P_=0.36, long-rank test). Group A, MMF (dashed line); group B, CsA (straight line).
Figure 3.
Pharmacokinetics of MPA and efficacy. (A) MPA exposure (MPA-AUC) estimated by three-point measuring demonstrating lack of correlation between drug dosage and drug concentration in serum. (B) Rate of relapses depending on MPA-exposition: The patients are divided in two groups according to an MPA-AUC <50 µg⋅h/ml or >50 µg⋅h/ml. The number of relapses (P<0.05) and the mean MPA-AUC are significantly different between groups (_P_=0.0001). (C) ROC curves computed for 3-month MPA-AUC values (_n_=43). Diagnostic sensitivities (true positives) are calculated for each individual AUC value as the fraction of patients with a recurrence to have lower values. The corresponding diagnostic specificities (false negatives) are calculated as the fraction of patients with no recurrence to have higher values. At a cutoff of 57.1 µg⋅h/ml, the MPA-AUC has a diagnostic sensitivity of 80.0% and a diagnostic specificity of 63.0% to discriminate relapsing from nonrelapsing patients (ROC-AUC=0.68, _P_=0.02). (D) Kaplan–Meier survival analysis comparing the time without relapse (cumulative sustained remission) in patients with low (dashed line) and high (straight line) MPA exposure (AUC) (_P_=0.03, long-rank test). (E) Linear regression and confidence intervals (dotted lines) of MPA drug exposure (MPA-AUC) and MPA predose concentration (MPA-C0).
Similar articles
- Cyclosporine versus mycophenolate mofetil for maintenance of remission of steroid-dependent nephrotic syndrome after a single infusion of rituximab.
Fujinaga S, Someya T, Watanabe T, Ito A, Ohtomo Y, Shimizu T, Kaneko K. Fujinaga S, et al. Eur J Pediatr. 2013 Apr;172(4):513-8. doi: 10.1007/s00431-012-1913-3. Epub 2012 Dec 28. Eur J Pediatr. 2013. PMID: 23271494 - [Mycophenolate mofetil in treatment of childhood nephrotic syndrome--preliminary report].
Kwinta-Rybicka J, Wilkosz K, Wierzchowska-Słowiacze EK, Ogarek I, Moczulska A, Stec Z, Pełkowska A, Sancewicz-Pach K, Pietrzyk JA. Kwinta-Rybicka J, et al. Przegl Lek. 2006;63 Suppl 3:44-8. Przegl Lek. 2006. PMID: 16898486 Clinical Trial. Polish. - Long-term outcome of Japanese children with complicated minimal change nephrotic syndrome treated with mycophenolate mofetil after cyclosporine.
Fujinaga S, Hirano D, Nishino T, Umeda C, Watanabe Y, Nakagawa M. Fujinaga S, et al. Pediatr Nephrol. 2019 Nov;34(11):2417-2421. doi: 10.1007/s00467-019-04339-y. Epub 2019 Aug 21. Pediatr Nephrol. 2019. PMID: 31435725 - Non-corticosteroid immunosuppressive medications for steroid-sensitive nephrotic syndrome in children.
Pravitsitthikul N, Willis NS, Hodson EM, Craig JC. Pravitsitthikul N, et al. Cochrane Database Syst Rev. 2013 Oct 29;(10):CD002290. doi: 10.1002/14651858.CD002290.pub4. Cochrane Database Syst Rev. 2013. PMID: 24166716 Updated. Review. - Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: current status and future directions.
Moudgil A, Bagga A, Jordan SC. Moudgil A, et al. Pediatr Nephrol. 2005 Oct;20(10):1376-81. doi: 10.1007/s00467-005-1964-z. Epub 2005 Jun 24. Pediatr Nephrol. 2005. PMID: 15977023 Review.
Cited by
- Nephrotoxicity in children with frequently relapsing nephrotic syndrome receiving long-term cyclosporine treatment.
Hamasaki Y, Komaki F, Ishikura K, Hamada R, Sakai T, Hataya H, Ogata K, Ando T, Honda M. Hamasaki Y, et al. Pediatr Nephrol. 2017 Aug;32(8):1383-1390. doi: 10.1007/s00467-017-3641-4. Epub 2017 Apr 4. Pediatr Nephrol. 2017. PMID: 28378029 - Positive role of rituximab in switching from cyclosporine to mycophenolate mofetil for children with high-dose steroid-dependent nephrotic syndrome.
Fujinaga S, Sakuraya K, Yamada A, Urushihara Y, Ohtomo Y, Shimizu T. Fujinaga S, et al. Pediatr Nephrol. 2015 Apr;30(4):687-91. doi: 10.1007/s00467-014-3034-x. Epub 2015 Jan 10. Pediatr Nephrol. 2015. PMID: 25576066 Clinical Trial. - Pharmacokinetics of mycophenolic acid in children with clinically stable idiopathic nephrotic syndrome receiving cyclosporine.
Hibino S, Nagai T, Yamakawa S, Ito H, Tanaka K, Uemura O. Hibino S, et al. Clin Exp Nephrol. 2017 Feb;21(1):152-158. doi: 10.1007/s10157-016-1267-7. Epub 2016 Apr 22. Clin Exp Nephrol. 2017. PMID: 27105859 - [Comparison of therapeutic effects of prednisone combined with mycophenolate mofetil versus cyclosporin A in children with steroid-resistant nephrotic syndrome].
Li ZH, Lin Z, Duan CR, Wu TH, Xun M, Zhang Y, Zhang L, Ding YF, Yin Y. Li ZH, et al. Zhongguo Dang Dai Er Ke Za Zhi. 2016 Feb;18(2):130-5. doi: 10.7499/j.issn.1008-8830.2016.02.007. Zhongguo Dang Dai Er Ke Za Zhi. 2016. PMID: 26903059 Free PMC article. Chinese. - Difficult-to-treat idiopathic nephrotic syndrome: established drugs, open questions and future options.
Kemper MJ, Valentin L, van Husen M. Kemper MJ, et al. Pediatr Nephrol. 2018 Oct;33(10):1641-1649. doi: 10.1007/s00467-017-3780-7. Epub 2017 Sep 6. Pediatr Nephrol. 2018. PMID: 28879428 Review.
References
- International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981 - PubMed
- Hodson EM, Willis NS, Craig JC: Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database Syst Rev (4): CD001533, 2007 - PubMed
- Tarshish P, Tobin JN, Bernstein J, Edelmann CM, Jr: Prognostic significance of the early course of minimal change nephrotic syndrome: Report of the International Study of Kidney Disease in Children. J Am Soc Nephrol 8: 769–776, 1997 - PubMed
- Hodson EM, Willis NS, Craig JC: Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev (1): CD002290, 2008 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources