The Ktr potassium transport system in Staphylococcus aureus and its role in cell physiology, antimicrobial resistance and pathogenesis - PubMed (original) (raw)
The Ktr potassium transport system in Staphylococcus aureus and its role in cell physiology, antimicrobial resistance and pathogenesis
Casey M Gries et al. Mol Microbiol. 2013 Aug.
Abstract
Potassium (K(+) ) plays a vital role in bacterial physiology, including regulation of cytoplasmic pH, turgor pressure and transmembrane electrical potential. Here, we examine the Staphylococcus aureus Ktr system uniquely comprised of two ion-conducting proteins (KtrB and KtrD) and only one regulator (KtrA). Growth of Ktr system mutants was severely inhibited under K(+) limitation, yet detectable after an extended lag phase, indicating the presence of a secondary K(+) transporter. Disruption of both ktrA and the Kdp-ATPase system, important for K(+) uptake in other organisms, eliminated regrowth in 0.1 mM K(+) , demonstrating a compensatory role for Kdp to the Ktr system. Consistent with K(+) transport mutations, S. aureus devoid of the Ktr system became sensitive to hyperosmotic conditions, exhibited a hyperpolarized plasma membrane, and increased susceptibility to aminoglycoside antibiotics and cationic antimicrobials. In contrast to other organisms, the S. aureus Ktr system was shown to be important for low-K(+) growth under alkaline conditions, but played only a minor role in neutral and acidic conditions. In a mouse competitive index model of bacteraemia, the ktrA mutant was significantly outcompeted by the parental strain. Combined, these results demonstrate a primary mechanism of K(+) uptake in S. aureus and a role for this system in pathogenesis.
© 2013 John Wiley & Sons Ltd.
Figures
Fig. 1
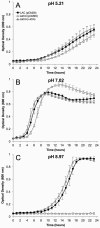
Growth of S. aureus Δ_ktrA_ mutant in 0.5 mM KCl at acidic, neutral, and alkaline pH. Wild-type S. aureus (solid circles) and the Δ_ktrA_ mutant (open triangles) were inoculated from overnight cultures to OD600=0.05 in K-CDM supplemented with 0.5 mM KCl. A and B. Growth in acidic (pH 5.21) or neutral (pH 7.02) conditions had minor effects on growth. C. At pH 8.97, the Δ_ktrA_ strain was unable to grow. A-C. Expression of ktrA from plasmid pCM28 in Δ_ktrA_ (solid triangles) fully complemented the growth defects in all conditions tested. Data represents the mean of at least two independent experiments, with ± standard deviation.
Fig. 2
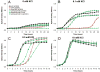
Growth of S. aureus Ktr mutants in low K+. Wild-type S. aureus (solid black circles) and mutant strains Δ_ktrA_ (open red triangles) and Δ_ktrB_Δ_ktrD_ (open blue squares) were inoculated from overnight cultures to OD600=0.05 in K-CDM supplemented with KCl to the desired concentration. A and B. Without supplemented KCl (A) and in 0.1 mM supplemented KCl (B), the Δ_ktrA_ and Δ_ktrB_Δ_ktrD_ strains had a severe growth delay compared to wild-type. C. In 0.5 mM KCl, the Δ_ktrA_ and Δ_ktrB_Δ_ktrD_ strains had an extended lag phase before entering a growth rate and yield similar to wild-type. D. With KCl supplemented to 10 mM, all stains grew equally. A-D. Expression of ktrA from plasmid pCM28 (solid red triangles) fully complemented the Δ_ktrA_ mutant growth defects, and expression of either ktrB (solid blue diamond) or ktrD (solid blue squares) from pCM28 fully complemented growth of the Δ_ktrB_Δ_ktrD_ strain in all conditions tested. Data represents the mean of three independent experiments, with ± standard deviation.
Fig. 3
Growth of S. aureus Kdp and Ktr mutants in low K+. Wildtype S. aureus (solid black circles) and mutant strains Δ_kdpFABC_ (open purple diamonds) and Δ_ktrA_Δ_kdpFABC_ (open green squares) were inoculated from overnight cultures to OD600=0.05 in K-CDM supplemented with KCl to the desired concentration. A and B. Without supplemented KCl (A) and in 0.1 mM supplemented KCl (B), the Δ_ktrA_Δ_kdpFABC_ double mutant was unable to grow, while the Δ_kdpFABC_ single mutant had only a slight growth defect compared to wild-type. The Δ_ktrA_ (open red triangles) mutant is shown for reference. C. In 0.5 mM added KCl, the Δ_ktrA_Δ_kdpFABC_ strain had a slower rate of growth than the single Δ_ktrA_ mutant. D. With KCl supplemented to 10 mM, all stains grew similarly. A-D. Expression of ktrA (solid green squares) from plasmid pCM28 fully complemented the Δ_ktrA_Δ_kdpFABC_ double mutant growth defects, and expression of kdpFABC (solid green diamonds) partially complemented growth of the Δ_ktrA_Δ_kdpFABC_ strain in all conditions. Data represents the mean of three independent experiments, with ± standard deviation.
Fig. 4
Ktr contributes to high osmotic growth. Wild-type (solid circles), Δ_ktrA_ (open triangles), and Δ_ktrA_(+ktrA) (solid triangles) strains were inoculated from overnight cultures to OD600=0.05 in K-CDM supplemented with KCl and either NaCl or Sorbitol to the desired concentration. A and B. Growth in high NaCl. The Δ_ktrA_ strain was unable to grow in K-CDM supplemented with 0.5 mM KCl and 400 mM NaCl (A), but recovered growth to near wild-type levels with added 10 mM KCl (B). C and D. Growth in high sorbitol. As an alternative solute, 750 mM sorbitol also severely delayed the growth of the Δ_ktrA_ mutant in 0.5 mM KCl (C), but was fully restored in 10 mM KCl (D). Expression of ktrA from a pCM28 fully complemented the growth defects of the Δ_ktrA_ mutant in all conditions. Data presented represent the mean of at least two independent experiments, with ± standard deviation.
Fig. 5
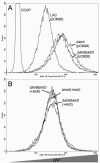
Ktr-mediated regulation of membrane potential. The cyanine dye DiOC2(3) was utilized to determine the role of the Ktr system in regulation of S. aureus resting electrical membrane potential. A. The Δ_ktrA_ and Δ_ktrB_Δ_ktrD_ mutant strains were hyperpolarized compared to wild-type. B. Hyperpolarization was decreased to near wild-type levels with pCM28-based expression of ktrA in the Δ_ktrA_ mutant and either ktrB or ktrD in the Δ_ktrB_Δ_ktrD_ strain. Results are representative of three independent experiments.
Fig. 6
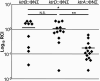
Ktr contributes for fitness in a mouse bacteremia model. C57BL/6 mice were infected with ∼3×106 CFU of ∼50% S. aureus JE2 and ∼50% ktrB::ΦNΣ (n=5 mice), ktrD::ΦNΣ (n=6 mice), or ktrA::ΦNΣ (n=7 mice). After 9 days, both kidneys were individually homogenized and bacterial burden of JE2 and ΦNΣ mutants were determined through viable cell count on TSA and TSA+Erm, respectively. RCI values were calculated by dividing the percentage of the ΦNΣ strain within each kidney by percentage of the ΦNΣ strain within the initial inoculum and plotted as an individual data point. Horizontal lines represents the RCI mean. The probability that significant differences in RCI are noted between ktrB::ΦNΣ and ktrA::ΦNΣ (*p=0.0128), ktrD::ΦNΣ and ktrA::ΦNΣ (**p=0.008), but not ktrB::ΦNΣ and ktrD::ΦNΣ (p=0.9716).
Similar articles
- KtrAB and KtrCD: two K+ uptake systems in Bacillus subtilis and their role in adaptation to hypertonicity.
Holtmann G, Bakker EP, Uozumi N, Bremer E. Holtmann G, et al. J Bacteriol. 2003 Feb;185(4):1289-98. doi: 10.1128/JB.185.4.1289-1298.2003. J Bacteriol. 2003. PMID: 12562800 Free PMC article. - Transcriptional profiling of Staphylococcus aureus during growth in 2 M NaCl leads to clarification of physiological roles for Kdp and Ktr K+ uptake systems.
Price-Whelan A, Poon CK, Benson MA, Eidem TT, Roux CM, Boyd JM, Dunman PM, Torres VJ, Krulwich TA. Price-Whelan A, et al. mBio. 2013 Aug 20;4(4):e00407-13. doi: 10.1128/mBio.00407-13. mBio. 2013. PMID: 23963175 Free PMC article. - Comparative analysis of kdp and ktr mutants reveals distinct roles of the potassium transporters in the model cyanobacterium Synechocystis sp. strain PCC 6803.
Nanatani K, Shijuku T, Takano Y, Zulkifli L, Yamazaki T, Tominaga A, Souma S, Onai K, Morishita M, Ishiura M, Hagemann M, Suzuki I, Maruyama H, Arai F, Uozumi N. Nanatani K, et al. J Bacteriol. 2015 Feb 15;197(4):676-87. doi: 10.1128/JB.02276-14. Epub 2014 Oct 13. J Bacteriol. 2015. PMID: 25313394 Free PMC article. - Metabolic control of virulence factor production in Staphylococcus aureus.
Rudra P, Boyd JM. Rudra P, et al. Curr Opin Microbiol. 2020 Jun;55:81-87. doi: 10.1016/j.mib.2020.03.004. Epub 2020 May 7. Curr Opin Microbiol. 2020. PMID: 32388086 Free PMC article. Review. - Natural Products That Target Virulence Factors in Antibiotic-Resistant Staphylococcus aureus.
Wu SC, Liu F, Zhu K, Shen JZ. Wu SC, et al. J Agric Food Chem. 2019 Dec 4;67(48):13195-13211. doi: 10.1021/acs.jafc.9b05595. Epub 2019 Nov 21. J Agric Food Chem. 2019. PMID: 31702908 Review.
Cited by
- Nucleotides as Bacterial Second Messengers.
Cancino-Diaz ME, Guerrero-Barajas C, Betanzos-Cabrera G, Cancino-Diaz JC. Cancino-Diaz ME, et al. Molecules. 2023 Dec 7;28(24):7996. doi: 10.3390/molecules28247996. Molecules. 2023. PMID: 38138485 Free PMC article. Review. - The impact of two-component sensorial network in staphylococcal speciation.
Rapun-Araiz B, Haag AF, Solano C, Lasa I. Rapun-Araiz B, et al. Curr Opin Microbiol. 2020 Jun;55:40-47. doi: 10.1016/j.mib.2020.02.004. Epub 2020 Mar 19. Curr Opin Microbiol. 2020. PMID: 32199334 Free PMC article. Review. - Augmented antibiotic resistance associated with cadmium induced alterations in Salmonella enterica serovar Typhi.
Kaur UJ, Preet S, Rishi P. Kaur UJ, et al. Sci Rep. 2018 Aug 24;8(1):12818. doi: 10.1038/s41598-018-31143-9. Sci Rep. 2018. PMID: 30143701 Free PMC article. - The PTSNtr-KdpDE-KdpFABC Pathway Contributes to Low Potassium Stress Adaptation and Competitive Nodulation of Sinorhizobium fredii.
Feng XY, Tian Y, Cui WJ, Li YZ, Wang D, Liu Y, Jiao J, Chen WX, Tian CF. Feng XY, et al. mBio. 2022 Jun 28;13(3):e0372121. doi: 10.1128/mbio.03721-21. Epub 2022 May 2. mBio. 2022. PMID: 35491828 Free PMC article. - An essential role for bacterial nitric oxide synthase in Staphylococcus aureus electron transfer and colonization.
Kinkel TL, Ramos-Montañez S, Pando JM, Tadeo DV, Strom EN, Libby SJ, Fang FC. Kinkel TL, et al. Nat Microbiol. 2016 Nov 28;2:16224. doi: 10.1038/nmicrobiol.2016.224. Nat Microbiol. 2016. PMID: 27892921 Free PMC article.
References
- Albright RA, Ibar JL, Kim CU, Gruner SM, Morais-Cabral JH. The RCK domain of the KtrAB K+ transporter: multiple conformations of an octameric ring. Cell. 2006;126:1147–1159. - PubMed
- Bakker EP. Alkali cation transport systems in prokaryotes. CRC Press; Boca Raton: 1993. p. 448.
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI083211/AI/NIAID NIH HHS/United States
- R01 AI038901/AI/NIAID NIH HHS/United States
- P01AI83211/AI/NIAID NIH HHS/United States
- R01AI038901/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases