Regulation of ubiquitin-dependent cargo sorting by multiple endocytic adaptors at the plasma membrane - PubMed (original) (raw)
Regulation of ubiquitin-dependent cargo sorting by multiple endocytic adaptors at the plasma membrane
Jonathan R Mayers et al. Proc Natl Acad Sci U S A. 2013.
Abstract
Endocytic protein trafficking is directed by sorting signals on cargo molecules that are recognized by cytosolic adaptor proteins. However, the steps necessary to segregate the variety of cargoes during endocytosis remain poorly defined. Using Caenorhabditis elegans, we demonstrate that multiple plasma membrane endocytic adaptors function redundantly to regulate clathrin-mediated endocytosis and to recruit components of the endosomal sorting complex required for transport (ESCRT) machinery to the cell surface to direct the sorting of ubiquitin-modified substrates. Moreover, our data suggest that preassembly of cargoes with the ESCRT-0 complex at the plasma membrane enhances the efficiency of downstream sorting events in the endolysosomal system. In the absence of a heterooligomeric adaptor complex composed of FCHO, Eps15, and intersectin, ESCRT-0 accumulation at the cell surface is diminished, and the degradation of a ubiquitin-modified cargo slows significantly without affecting the rate of its clathrin-mediated internalization. Consistent with a role for the ESCRT machinery during cargo endocytosis, we further show that the ESCRT-0 complex accumulates at a subset of clathrin-coated pits on the surface of human cells. Our findings suggest a unique mechanism by which ubiquitin-modified cargoes are sequestered into the endolysosomal pathway.
Keywords: clathrin; multivesicular endosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
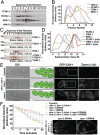
Fig. 1.
FCHO-1, EHS-1, and ITSN-1 are plasma membrane-specific adaptor proteins. (A) Whole-worm extracts generated from control (N2) animals and mutant strains were separated by SDS/PAGE and immunoblotted by using affinity-purified antibodies as indicated. (B) Embryos isolated from control hermaphrodites (N2) and strains lacking FCHO-1 (Top Right) or ITSN-1 (Bottom Right) were fixed and stained by using antibodies directed against each protein. (C) Control embryos were fixed and stained by using antibodies directed against RAB-5 (Cy2; Left) and ITSN-1 (Cy3; Center). A 7× zoom of a boxed region is also provided (Right). (D) Control (Upper) and VPS-32–depleted embryos (Lower) stably expressing a GFP fusion to clathrin light chain (GFP-CLIC-1) were imaged by using swept-field confocal optics. Maximum intensity projections of four cortical sections (2 μm thick), taken ∼9 μm from the coverslip, are shown. (E) Following background subtraction, the total integrated intensity of GFP-CLIC-1 at the cell-cell junction of two-cell stage embryos was calculated (within several 1-μm2 regions) in control and vps-32(RNAi) embryos. Representative images are shown (n = 12 embryos for each condition). **, significant difference (P < 0.01). (F) Embryos expressing GFP-CAV-1 were depleted of VPS-32 by using RNAi and subsequently fixed and stained by using antibodies directed against ITSN-1 and GFP. (Scale bars: 10 μm.)
Fig. 2.
FCHO-1, EHS-1, and ITSN-1 form a stable complex that functions in the trafficking of ubiquitin-modified cargoes to the lysosome for degradation. (A) An embryo extract was separated by gel filtration chromatography, and eluted fractions were immunoblotted by using antibodies directed against ITSN-1, FCHO-1, EHS-1, and APA-2. Based on densitometry measurements, peak fractions were identified for each protein (boxed regions). (B) Graphical representation of densitometry measurements conducted on immunoblots shown in A. (C) Recombinant ITSN-1, EHS-1, and FCHO-1 were applied individually or as a mixture onto a gel filtration column, and eluted fractions were separated by SDS/PAGE before silver staining. The Stokes radius for each protein was calculated based on the elution profiles of characterized standards. (D) Recombinant ITSN-1, EHS-1, and FCHO-1 were applied individually or as a mixture onto glycerol gradients (10–30%), which were centrifuged and fractionated. Each fraction was separated by SDS/PAGE and silver stained. Densitometry measurements, which are shown in a graphical representation, were used to define the sedimentation value of each protein (boxed region). (E) Immobilized control, ehs-1;itsn-1;fcho-1 triple mutant animals, and stam-1 single mutant animals expressing GFP-CAV-1 were imaged by using DIC (Left) and swept-field confocal optics (Right). Cartoons highlighting the number of cells contained within each embryo observed in utero are provided (Center). Additionally, a 1.6× zoom of a boxed region within the uterus of each animal is also shown. (F) The rates of GFP-CAV-1 endocytosis from the plasma membrane following ovulation were analyzed in control (N2) or ehs-1;itsn-1;fcho-1 triple mutant embryos by measuring the loss of cell surface fluorescence over time in utero (n = 5 for each condition). In some cases, animals were depleted of epsin (epn-1), APA-2, or clathrin heavy chain (chc-1) as indicated. A comparison of GFP:CAV-1 localization in one cell stage ehs-1;itsn-1;fcho-1 triple mutant embryos (during metaphase) following APA-2 or EPN-1 depletion is also shown (Right). (Scale bars: 10 μm.)
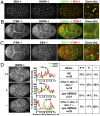
Fig. 3.
ESCRT-0 localization to the plasma membrane depends on the presence of multiple endocytic adaptors. (A) One cell stage embryos were fixed and stained with directly labeled antibodies against HGRS-1 (Cy2) and EEA-1 (Cy3). Images (∼4 μm from the coverslip) were acquired by using swept-field confocal optics. A 3× zoom of the boxed region is also shown (Right). (B and C) Four cell-stage embryos were fixed and stained with labeled antibodies against HGRS-1 (Cy2), EEA-1 (Cy2), or ITSN-1 (Cy3). Images (∼9 μm from the coverslip) were acquired by using swept-field confocal optics. A 3× zoom of boxed regions is also provided (Right). (D) Four cell stage embryos expressing a GFP fusion to a CAAX motif (
Fig. S5_A_
) were fixed and stained by using antibodies directed against HGRS-1, and plasma membrane localization was quantified based on fluorescence intensity by using a linescan analysis (highlighted in each image). Peak fluorescence intensity of the GFP signal was used to define the localization of the plasma membrane. A table showing the relative intensity of HGRS-1 on the plasma membrane in different mutant backgrounds is shown on Right (++, high level of HGRS-1; +, low level of HGRS-1; −, an absence of HGRS-1). (Scale bars: A_–_D, 10 μm; A_–_C Inset, 2 μm.)
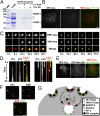
Fig. 4.
ESCRT-0 associates with clathrin-coated pits at the plasma membrane. (A) Coflotation assays were conducted by using recombinant ESCRT-0 and liposomes containing either 70% phosphatidylcholine (PC) and 30% phosphatidylethanolamine (PE); 69% PC, 30% PE, and 1% PI3P; or 15% PS, 55% PC, and 30% PE. Asterisks highlight the presence of bacterial contaminants. (B) HeLa cells expressing RFP-clathrin light chain (LCa) and YFP-Hrs were imaged by using TIRF microscopy. (C) Individual sites of clathrin-mediated endocytosis were imaged over time by using TIRF microscopy. Merged panels showing both RFP-LCa and YFP-Hrs localization are shown (Bottom). (D) Kymographs showing the fate of RFP-LCa and YFP-Hrs at clathrin-coated pits over time. Arrows highlight the timing of Hrs appearance (Left) or disappearance (Right). (E) HeLa cells expressing RFP-clathrin light chain (LCa) and GFP-Mvb12B were imaged by using TIRF microscopy. (F) HeLa cells stably expressing YFP-Hrs were pretreated with dynasore (200 μM) for 1 h and subsequently pulse-labeled with fluorescent EGF (10 ng/mL) for 2 min before fixation and imaging. (G) Model highlighting a role for ESCRT-0 in the capture of ubiquitin-modified cargoes at a subset of clathrin-coated pits. (Scale bars: B and E, 10 μm; C and D, 2 μm; F, 5 μm.)
Similar articles
- ESCRT-dependent protein sorting is required for the viability of yeast clathrin-mediated endocytosis mutants.
Hoban K, Lux SY, Poprawski J, Zhang Y, Shepherdson J, Castiñeira PG, Pesari S, Yao T, Prosser DC, Norris C, Wendland B. Hoban K, et al. Traffic. 2020 Jun;21(6):430-450. doi: 10.1111/tra.12731. Traffic. 2020. PMID: 32255230 Free PMC article. - Ist1 regulates ESCRT-III assembly and function during multivesicular endosome biogenesis in Caenorhabditis elegans embryos.
Frankel EB, Shankar R, Moresco JJ, Yates JR 3rd, Volkmann N, Audhya A. Frankel EB, et al. Nat Commun. 2017 Nov 13;8(1):1439. doi: 10.1038/s41467-017-01636-8. Nat Commun. 2017. PMID: 29129923 Free PMC article. - Ubiquitin recognition in endocytic trafficking - with or without ESCRT-0.
Mosesso N, Nagel MK, Isono E. Mosesso N, et al. J Cell Sci. 2019 Aug 15;132(16):jcs232868. doi: 10.1242/jcs.232868. J Cell Sci. 2019. PMID: 31416855 Review. - Ubiquitin initiates sorting of Golgi and plasma membrane proteins into the vacuolar degradation pathway.
Scheuring D, Künzl F, Viotti C, Yan MS, Jiang L, Schellmann S, Robinson DG, Pimpl P. Scheuring D, et al. BMC Plant Biol. 2012 Sep 12;12:164. doi: 10.1186/1471-2229-12-164. BMC Plant Biol. 2012. PMID: 22970698 Free PMC article. - Decoding ubiquitin sorting signals for clathrin-dependent endocytosis by CLASPs.
Traub LM, Lukacs GL. Traub LM, et al. J Cell Sci. 2007 Feb 15;120(Pt 4):543-53. doi: 10.1242/jcs.03385. J Cell Sci. 2007. PMID: 17287393 Review.
Cited by
- Liquid-like protein interactions catalyse assembly of endocytic vesicles.
Day KJ, Kago G, Wang L, Richter JB, Hayden CC, Lafer EM, Stachowiak JC. Day KJ, et al. Nat Cell Biol. 2021 Apr;23(4):366-376. doi: 10.1038/s41556-021-00646-5. Epub 2021 Apr 5. Nat Cell Biol. 2021. PMID: 33820972 Free PMC article. - Decoding the Role of Extracellular Vesicles in Liver Diseases.
Deng F, Magee N, Zhang Y. Deng F, et al. Liver Res. 2017 Sep;1(3):147-155. doi: 10.1016/j.livres.2017.11.003. Epub 2017 Dec 7. Liver Res. 2017. PMID: 29552373 Free PMC article. - Growth factor stimulation promotes multivesicular endosome biogenesis by prolonging recruitment of the late-acting ESCRT machinery.
Quinney KB, Frankel EB, Shankar R, Kasberg W, Luong P, Audhya A. Quinney KB, et al. Proc Natl Acad Sci U S A. 2019 Apr 2;116(14):6858-6867. doi: 10.1073/pnas.1817898116. Epub 2019 Mar 20. Proc Natl Acad Sci U S A. 2019. PMID: 30894482 Free PMC article. - NECAPs are negative regulators of the AP2 clathrin adaptor complex.
Beacham GM, Partlow EA, Lange JJ, Hollopeter G. Beacham GM, et al. Elife. 2018 Jan 18;7:e32242. doi: 10.7554/eLife.32242. Elife. 2018. PMID: 29345618 Free PMC article. - A Drosophila model of neuronal ceroid lipofuscinosis CLN4 reveals a hypermorphic gain of function mechanism.
Imler E, Pyon JS, Kindelay S, Torvund M, Zhang YQ, Chandra SS, Zinsmaier KE. Imler E, et al. Elife. 2019 Oct 30;8:e46607. doi: 10.7554/eLife.46607. Elife. 2019. PMID: 31663851 Free PMC article.
References
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438(7068):590–596. - PubMed
- McNiven MA, Thompson HM. Vesicle formation at the plasma membrane and trans-Golgi network: The same but different. Science. 2006;313(5793):1591–1594. - PubMed
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12(8):517–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P41 GM103533/GM/NIGMS NIH HHS/United States
- P41RR011823/RR/NCRR NIH HHS/United States
- R01 GM088151/GM/NIGMS NIH HHS/United States
- P41 RR011823/RR/NCRR NIH HHS/United States
- 1R01GM088151-01A1/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous