Cytoplasmic TERT Associates to RNA Granules in Fully Mature Neurons: Role in the Translational Control of the Cell Cycle Inhibitor p15INK4B - PubMed (original) (raw)
Cytoplasmic TERT Associates to RNA Granules in Fully Mature Neurons: Role in the Translational Control of the Cell Cycle Inhibitor p15INK4B
Francesca Iannilli et al. PLoS One. 2013.
Abstract
The main role of Telomerase Reverse Transcriptase (TERT) is to protect telomere length from shortening during cell division. However, recent works have revealed the existence of a pool of TERT associated to mitochondria, where it plays a role in survival. We here show that in fully differentiated neurons the largest pool of cytoplasmic TERT associates to TIA1 positive RNA granules, where it binds the messenger RNA of the cyclin kinase inhibitor p15INK4B. Upon stress, p15INK4B and TERT dissociate and p15INK4B undergoes efficient translation, allowing its pro-survival function. These results unveil another mechanism implicated in the survival of fully differentiated neurons.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
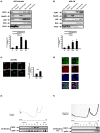
Figure 1. Neuronal TERT is enriched in ribosome-containing compartments in vivo. a)-b)
Western blot analysis of TERT localization in subcellular fractions from the brains of 23 month-old mice and rats. Hsp60, RibophorinI, p58 and Histone3 are markers of, respectively, mitochondria, ribosomes, microsomes and nuclei. TERT is clearly enriched in the microsome and ribosomal fractions in old mouse brain. Bar graph reflects these differences (mean ± s.d. of four different mice, mean ± s.d. of three different rats, *p<0.05). c) Representative confocal images of 23 DIV hippocampal neurons stained for TERT (red) and counterstained with MTT, mitochondrial selective dye (green) and p58 (green), microsomal marker. Bar: 10 µm. Bar graph on the right: quantification of soluble TERT in mitochondria and microsomal fractions; bars represent the mean ± s.d. of three different cultures (*p<0.05). d) Representative confocal images of brain slices from 23 months old mice stained with TERT (green), p58 (red) and DAPI (blue). Note the clear cytoplasmic distribution of TERT in the neurons of old mice brain and its concentration in the juxta-nuclear region, coinciding with the enrichment in p58 positive structures (n = 2). Scale bar: 20 µm. e) TERT partitioning in polysome gradients from total brain of 23 month-old mice. Note the abundance of TERT in fractions 6–7, corresponding to monosomes and the initiation translational complex (n = 3). f) Polysome gradient of old mice brain extracts treated with 1 mM Puromycin. Under this treatment, TERT is displaced to the mRNP fractions. The western blots below each gradient reveal the distribution of TERT and rpS6, used as marker for ribosome-containing fractions (n = 3).
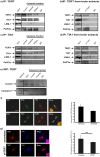
Figure 2. TERT associates to TIA1 –positive granules.
a) Western blot analysis of TERT immunoprecipitated proteins in extracts from hippocampal neurons maintained in vitro for 10 DIV, under basal stress conditions (control) or stressed with arsenite. Note that the two SG markers (TIA1 and P-elF2α) are precipitated whereas LSM-1, a component of PBs is not. RNase treatment does not affect TERT-TIA1 binding (n = 4). b) Western blot analysis of TERT immunoprecipitated proteins in extracts from old mice. As in the in vitro experiments, TIA1 and P-elF2α are precipitated whereas LSM-1 is not (n = 3). c) Western blot analysis of TIA1 immunoprecipitated proteins in extracts from hippocampal neurons maintained in vitro for 10 DIV, under basal stress conditions (control) or stressed with arsenite. Again, RNase treatment does not affect TERT-TIA1 binding (n = 4). d) Western blot analysis of TIA1 immunoprecipitated proteins in extracts from old mice. Note that TIA1 and P-elF2α are precipitated whereas LSM-1 is not (n = 3). e) The known TIA1 target ß-actin mRNA is amplified in RNA purified from TERT immunoprecipitate, whereas another known TIA1 target, Caspase-7, is not. The first lane corresponds to the markers (n = 2). f) Confocal microscopy images of neurons double labeled TERT (green)-TIA1 (red) (upper row) and TERT (green)-PABP (red) (lower row). Numerous foci of colocalization exist (quantified in the bar graph: mean ± the s.d. from three different experiments). Scale bar: 10 µm. g) Confocal microscopy images of neurons infected with scrambled or shTERT, stained for TERT (red) and counterstained for TIA1 (blue). The reduction in TIA1 labeling is not significant (mean ± the s.d. from three different experiments). Scale bar: 10 µm.
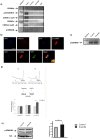
Figure 3. TERT granules contain the mRNA encoding the pro-survival cyclin inhibitor p15INK4B.
a) TERT RNA-immunoprecipitation from cells under control or arsenite-induced stress: immunoprecipitated RNA was used for RT-PCR with specific primers for cell cycle regulators. Note that the only positive amplification product corresponds to the p15INK4B messenger, in the control but not stressed neurons (n = 2). b) Upper panel. p15INK4B in situ hybridization, negative control (anti-sense) and p15INK4B specific probe. Only the specific probe gives a signal, in the nucleus (DAPI positive) and in the cytoplasm. Lower panel. p15INK4B in situ hybridization (red) together with TERT immunofluorescence microscopy (green); nuclear labeling with DAPI (blue). Colocalization is evident in the perinuclear region (arrows in overlay image, “merge”) (n = 3). c) p15INK4B mRNA levels in 10 DIV hippocampal neurons in culture, under control or arsenite treatment. Arsenite does not result in degradation of the messenger (n = 3). d) Representative A254 gradient profile of control (Ctr) and arsenite stressed neurons (Ars); translational efficiency of p15INK4B mRNA was normalized to Histone 3 and β-actin (beta-actin) mRNA, as measured by RT-qPCR assay, using the following algorithm: 2-[ΔCt(P)- ΔCt(mRNPs)]. Stress induces p15INK4B translocation to the polysomes, reflecting higher translation. Standard errors are shown (n = 3). e) Western blot analysis of p15INK4B from 10 DIV hippocampal neurons in culture, in control and in neurons treated with arsenite. Tubulin is used as loading control. Note that arsenite increases the levels of p15INK4B. Bar graph on the right is the quantification of this experiment (means ± the s.d. of three different cultures; *p<0.05).
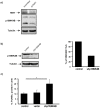
Figure 4. TERT exerts its anti-apoptotic role through regulation of p15INK4B messenger,
a**)** Western blot analysis of TERT and p15INK4B levels in 10 DIV hippocampal neurons infected with scrambled or shTERT; Tubulin is used as loading control. Note that p15INK4B levels are reduced by TERT downregulation (n = 2). b) Western blot analysis of p15INK4B levels in control 10 DIV hippocampal neurons and infected with p15INK4B shRNA. The reduction in protein content is more than 50% (bar graph on the right, n = 2). c) Tunel assay of 10 DIV hippocampal neurons under control conditions (control) and after infection with an empty vector (vector) or with the shRNA for p15INK4B (shp15INK4B). The bar graph illustrates the significant cell death under this last condition (mean ± the s.d. of three different cultures; * p<0.05).
Similar articles
- Telomerase reverse transcriptase upregulation attenuates astrocyte proliferation and promotes neuronal survival in the hypoxic-ischemic rat brain.
Qu Y, Duan Z, Zhao F, Wei D, Zhang J, Tang B, Li J, Yang C, Mu D. Qu Y, et al. Stroke. 2011 Dec;42(12):3542-50. doi: 10.1161/STROKEAHA.111.626325. Epub 2011 Sep 22. Stroke. 2011. PMID: 21940960 Retracted. - Translation of p15.5INK4B, an N-terminally extended and fully active form of p15INK4B, is initiated from an upstream GUG codon.
Fuxe J, Raschperger E, Pettersson RF. Fuxe J, et al. Oncogene. 2000 Mar 23;19(13):1724-8. doi: 10.1038/sj.onc.1203496. Oncogene. 2000. PMID: 10763830 - Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-beta2.
Joyce NC, Harris DL, Mello DM. Joyce NC, et al. Invest Ophthalmol Vis Sci. 2002 Jul;43(7):2152-9. Invest Ophthalmol Vis Sci. 2002. PMID: 12091410 - Non-canonical Functions of Telomerase Reverse Transcriptase: Emerging Roles and Biological Relevance.
Thompson CAH, Wong JMY. Thompson CAH, et al. Curr Top Med Chem. 2020;20(6):498-507. doi: 10.2174/1568026620666200131125110. Curr Top Med Chem. 2020. PMID: 32003692 Review. - The structure and function of telomerase reverse transcriptase.
Autexier C, Lue NF. Autexier C, et al. Annu Rev Biochem. 2006;75:493-517. doi: 10.1146/annurev.biochem.75.103004.142412. Annu Rev Biochem. 2006. PMID: 16756500 Review.
Cited by
- Importin 7 and Nup358 promote nuclear import of the protein component of human telomerase.
Frohnert C, Hutten S, Wälde S, Nath A, Kehlenbach RH. Frohnert C, et al. PLoS One. 2014 Feb 20;9(2):e88887. doi: 10.1371/journal.pone.0088887. eCollection 2014. PLoS One. 2014. PMID: 24586428 Free PMC article. - Melatonin Reduces Neuroinflammation and Improves Axonal Hypomyelination by Modulating M1/M2 Microglia Polarization via JAK2-STAT3-Telomerase Pathway in Postnatal Rats Exposed to Lipopolysaccharide.
Zhou Q, Lin L, Li H, Wang H, Jiang S, Huang P, Lin Q, Chen X, Deng Y. Zhou Q, et al. Mol Neurobiol. 2021 Dec;58(12):6552-6576. doi: 10.1007/s12035-021-02568-7. Epub 2021 Sep 28. Mol Neurobiol. 2021. PMID: 34585328 Free PMC article. - The role of telomerase protein TERT in Alzheimer's disease and in tau-related pathology in vitro.
Spilsbury A, Miwa S, Attems J, Saretzki G. Spilsbury A, et al. J Neurosci. 2015 Jan 28;35(4):1659-74. doi: 10.1523/JNEUROSCI.2925-14.2015. J Neurosci. 2015. PMID: 25632141 Free PMC article. - Non-canonical Roles of Telomerase: Unraveling the Imbroglio.
Ségal-Bendirdjian E, Geli V. Ségal-Bendirdjian E, et al. Front Cell Dev Biol. 2019 Dec 10;7:332. doi: 10.3389/fcell.2019.00332. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31911897 Free PMC article. Review. - Telomerase Biology Associations Offer Keys to Cancer and Aging Therapeutics.
Smith-Sonneborn J. Smith-Sonneborn J. Curr Aging Sci. 2020;13(1):11-21. doi: 10.2174/1874609812666190620124324. Curr Aging Sci. 2020. PMID: 31544708 Free PMC article. Review.
References
- HAyflick L, Moorhead PS (1961) The serial cultivation of human diploid cell strains. Exp Cell Res 25: 585–621. - PubMed
- Olovnikov AM (1973) A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41: 181–190. - PubMed
- Fortini P, Pascucci B, Parlanti E, D’Errico M, Simonelli V, et al. (2003) 8-Oxoguanine DNA damage: at the crossroad of alternative repair pathways. Mutat Res 531: 127–139. - PubMed
- Wright WE, Shay JW (2005) Telomere biology in aging and cancer. J Am Geriatr Soc 53: S292–294 doi:10.1111/j.1532-5415.2005.53492.x - DOI - PubMed
- Sodero AO, Trovò L, Iannilli F, Van Veldhoven P, Dotti CG, et al. (2011) Regulation of tyrosine kinase B activity by the Cyp46/cholesterol loss pathway in mature hippocampal neurons: relevance for neuronal survival under stress and in aging. J Neurochem 116: 747–755 doi:10.1111/j.1471-4159.2010.07079.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was partially supported by Flanders Fund for Scientific Research (FWO G 0.666.10N), NEUROBRAINNET IAP 7/16, Flemish Government Methusalem Grant, Spanish Ministry of Science and Innovation Ingenio-Consolider CSD2010-00064 and SAF2010-14906. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous