Somatic mutation, genomic variation, and neurological disease - PubMed (original) (raw)
Review
Somatic mutation, genomic variation, and neurological disease
Annapurna Poduri et al. Science. 2013.
Abstract
Genetic mutations causing human disease are conventionally thought to be inherited through the germ line from one's parents and present in all somatic (body) cells, except for most cancer mutations, which arise somatically. Increasingly, somatic mutations are being identified in diseases other than cancer, including neurodevelopmental diseases. Somatic mutations can arise during the course of prenatal brain development and cause neurological disease-even when present at low levels of mosaicism, for example-resulting in brain malformations associated with epilepsy and intellectual disability. Novel, highly sensitive technologies will allow more accurate evaluation of somatic mutations in neurodevelopmental disorders and during normal brain development.
Figures
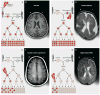
Fig. 1. Inherited, de novo, and somatic mutations causing neurological disease
(A) A heterozygous mutation is inherited from one parent. This mechanism is typical of autosomal dominant epilepsy. In this example, the mutation originally presented in the mother, whose oocytes in turn carry the mutation. (The mutation arose during gametogenesis in one of the parents of the mother, top left.) It is present in the zygote and thus all cells of the affected child. (B) This axial T1-weighted image from a MRI study of a patient with inherited epilepsy appears normal. Individuals with dominantly inherited epilepsies caused by mutations in genes encoding ion channels, for example, have normal neuroimaging studies despite every cell carrying a mutation. (C) A de novo mutation may arise sporadically during gametogenesis, in this case spermatogenesis. This mechanism of mutation would be typical of a de novo mutation in the gene SCN1A associated with severe myoclonic epilepsy of infancy or LIS1 associated with lissencephaly. Even though every cell in the individual carries the mutation, the predominant effects of the mutation depend on the distribution of gene expression; in these examples, the brain is primarily affected. (D) An axial T2-weighted MRI image shows the severe gyral simplification—more pronounced posteriorly (the bottom of the figure)—that is associated with mutations in the gene LIS1. (E) An early post-zygotic mutation results in a mutation present in most or all tissues of the organism (including the leukocytes, which are generally assayed for clinical genetic testing) but in a mosaic fashion, with only a portion of all cells in each tissue harboring the mutation. This pattern, illustrated by the axial T1-weighted image in (F), has been observed in mosaic cases of double cortex syndrome involving the gene DCX. Visible is the extra band of gray matter underlying the normal-appearing outer aspect of the cerebral cortex. Because DCX is required for normal migration of neurons from the ventricular region deep in the brain to the superficial cortex, the cells carrying the DCX mutation only migrate about halfway to the cortex and then arrest their migration. (G) A late post-zygotic mutation will be present in only certain tissues in a mosaic fashion, in this case apparently in half of the brain. This is the pattern observed in some cases of HMG with somatic mosaic point mutations in AKT3 and other related genes and somatic mosaic increase of copy number of chromosome 1q. (H) This axial T2-weighted MRI image shows right-sided HMG, characterized here by enlargement of the right hemisphere, abnormally thick and dark-appearing gray matter anteriorly, heterotopic periventricular gray matter, and abnormal white matter signal in the right hemisphere. (R, right; L, left).
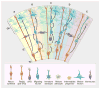
Fig. 2. Cortical development_—_origins of pyramidal neurons and astrocytes in the cerebral cortex
(A) A neuroepithelial cell (red) at the ventricular zone serves as progenitor for both a pyramidal neuron (green-blue) as well as a radial glial cell (gold). (B) A newly differentiated neuron (blue) migrates along a radial glial process. (C) Neurons (blue) continue to migrate as intermediate progenitor cells (small yellow) form. (D) Intermediate progenitor cells begin to generate neurons (blue). (E) The progenitor cells in the ventricular zone begin to give rise to astrocytes (dark green). Interneurons (purple) generated elsewhere migrate tangentially. CP, cortical plate; IZ, intermediate zone; VZ, ventricular zone. The VZ early in development has a thickness of ~10 cell bodies (50 to 100 μm). The CP ranges in thickness from two to three cell bodies at the earliest stages of development, eventually forming a mature cerebral cortex that is 2 to 4 mm thick.
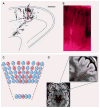
Fig. 3. Focal cortical dysplasia: a clonal-appearing brain lesion suspected to be caused by somatic mutation in a progenitor cell
(A) A camera lucida drawing shows a radial cluster from the developing ferret brain, labeled at E29 and studied at P8 (scale bar, 500 μm) [from (68)]. The arrow indicates the region enlarged in (B). The accompanying photograph (B) shows an enlarged view of retro-virally labeled migrating pyramidal neurons at the same stage. (C) A schematic depicts the predicted effects of a somatic mutation affecting a progenitor cell in the VZ, producing a funnel-shaped lesion emanating from the ventricle. Shown are offspring (red) of the progenitor that experienced a mutation and are partially interspersed with normal cells (blue). (D) This axial T2-weighted MRI of the brain of a patient with focal epilepsy shows a focal cortical dysplasia in the left frontal region. In this image, normal gray matter appears gray, and normal white matter appears black. In the expanded view of the lesion, outlined in the smaller region by the blue box, the MRI shows a wedge-shaped abnormality roughly bounded by red dashed lines. The lesion consists of abnormal white matter signal (normally black, but in the lesion light gray in the region between the dashed lines) and thickened gray matter. The boundary between the gray matter and the white matter is blurred in this focal region as compared with the rest of the brain. In contrast, the normal regions surrounding the focal cortical dysplasia show gray matter of appropriate thickness, a sharp distinction between the gray matter and the white matter, and appropriate (black) signal in the white matter. [The MRI image is courtesy of Dr. A. James Barkovich, Department of Radiology, University of California, San Francisco]
Similar articles
- Somatic mutations in cerebral cortical malformations.
Jamuar SS, Lam AT, Kircher M, D'Gama AM, Wang J, Barry BJ, Zhang X, Hill RS, Partlow JN, Rozzo A, Servattalab S, Mehta BK, Topcu M, Amrom D, Andermann E, Dan B, Parrini E, Guerrini R, Scheffer IE, Berkovic SF, Leventer RJ, Shen Y, Wu BL, Barkovich AJ, Sahin M, Chang BS, Bamshad M, Nickerson DA, Shendure J, Poduri A, Yu TW, Walsh CA. Jamuar SS, et al. N Engl J Med. 2014 Aug 21;371(8):733-43. doi: 10.1056/NEJMoa1314432. N Engl J Med. 2014. PMID: 25140959 Free PMC article. - [A male case of subcortical band heterotopia with somatic mosaicism of DCX mutation].
Igarashi A, Kawatani M, Ohta G, Kometani H, Ohshima Y, Kato M. Igarashi A, et al. No To Hattatsu. 2013 Sep;45(5):371-4. No To Hattatsu. 2013. PMID: 24205692 Japanese. - Paternal transmission of subcortical band heterotopia through DCX somatic mosaicism.
Moreira I, Bastos-Ferreira R, Silva J, Ribeiro C, Alonso I, Chaves J. Moreira I, et al. Seizure. 2015 Feb;25:62-4. doi: 10.1016/j.seizure.2014.12.005. Epub 2014 Dec 19. Seizure. 2015. PMID: 25645638 No abstract available. - [Epileptogenic brain malformations: radiological and clinical presentation and indications for genetic testing].
Bahi-Buisson N, Boddaert N, Saillour Y, Souville I, Poirier K, Léger PL, Castelnau L, Plouin P, Carion N, Beldjord C, Chelly J. Bahi-Buisson N, et al. Rev Neurol (Paris). 2008 Dec;164(12):995-1009. doi: 10.1016/j.neurol.2008.04.006. Epub 2008 Jun 9. Rev Neurol (Paris). 2008. PMID: 18808783 Review. French. - Lissencephaly: Update on diagnostics and clinical management.
Koenig M, Dobyns WB, Di Donato N. Koenig M, et al. Eur J Paediatr Neurol. 2021 Nov;35:147-152. doi: 10.1016/j.ejpn.2021.09.013. Epub 2021 Oct 7. Eur J Paediatr Neurol. 2021. PMID: 34731701 Review.
Cited by
- Investigating somatic aneuploidy in the brain: why we need a new model.
Rosenkrantz JL, Carbone L. Rosenkrantz JL, et al. Chromosoma. 2017 Jun;126(3):337-350. doi: 10.1007/s00412-016-0615-4. Epub 2016 Sep 16. Chromosoma. 2017. PMID: 27638401 Free PMC article. Review. - Dynamic self-guiding analysis of Alzheimer's disease.
Kurakin A, Bredesen DE. Kurakin A, et al. Oncotarget. 2015 Jun 10;6(16):14092-122. doi: 10.18632/oncotarget.4221. Oncotarget. 2015. PMID: 26041885 Free PMC article. Review. - High-level gonosomal mosaicism for a pathogenic non-coding CNV deletion of the lung-specific FOXF1 enhancer in an unaffected mother of an infant with ACDMPV.
Yıldız Bölükbaşı E, Karolak JA, Szafranski P, Gambin T, Willard N, Abman SH, Galambos C, Kinsella JP, Stankiewicz P. Yıldız Bölükbaşı E, et al. Mol Genet Genomic Med. 2022 Nov;10(11):e2062. doi: 10.1002/mgg3.2062. Epub 2022 Sep 20. Mol Genet Genomic Med. 2022. PMID: 36124617 Free PMC article. - One brain, many genomes.
Evrony GD. Evrony GD. Science. 2016 Nov 4;354(6312):557-558. doi: 10.1126/science.aak9761. Science. 2016. PMID: 27811258 Free PMC article. No abstract available. - Somatic mutations in the human brain: implications for psychiatric research.
Nishioka M, Bundo M, Iwamoto K, Kato T. Nishioka M, et al. Mol Psychiatry. 2019 Jun;24(6):839-856. doi: 10.1038/s41380-018-0129-y. Epub 2018 Aug 7. Mol Psychiatry. 2019. PMID: 30087451 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
- R01 NS032457/NS/NINDS NIH HHS/United States
- R01 MH083565/MH/NIMH NIH HHS/United States
- T32 GM007226/GM/NIGMS NIH HHS/United States
- RC2 MH089952/MH/NIMH NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- R01 NS079277/NS/NINDS NIH HHS/United States
- 1RC2MH089952/MH/NIMH NIH HHS/United States
- R37 NS035129/NS/NINDS NIH HHS/United States
- R01 NS035129/NS/NINDS NIH HHS/United States
- K23NS069784/NS/NINDS NIH HHS/United States
- T32GM007726-35/GM/NIGMS NIH HHS/United States
- T32GM007753/GM/NIGMS NIH HHS/United States
- K23 NS069784/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical