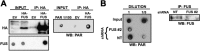The RNA-binding protein fused in sarcoma (FUS) functions downstream of poly(ADP-ribose) polymerase (PARP) in response to DNA damage - PubMed (original) (raw)
The RNA-binding protein fused in sarcoma (FUS) functions downstream of poly(ADP-ribose) polymerase (PARP) in response to DNA damage
Adam S Mastrocola et al. J Biol Chem. 2013.
Abstract
The list of factors that participate in the DNA damage response to maintain genomic stability has expanded significantly to include a role for proteins involved in RNA processing. Here, we provide evidence that the RNA-binding protein fused in sarcoma/translocated in liposarcoma (FUS) is a novel component of the DNA damage response. We demonstrate that FUS is rapidly recruited to sites of laser-induced DNA double-strand breaks (DSBs) in a manner that requires poly(ADP-ribose) (PAR) polymerase activity, but is independent of ataxia-telangiectasia mutated kinase function. FUS recruitment is mediated by the arginine/glycine-rich domains, which interact directly with PAR. In addition, we identify a role for the prion-like domain in promoting accumulation of FUS at sites of DNA damage. Finally, depletion of FUS diminished DSB repair through both homologous recombination and nonhomologous end-joining, implicating FUS as an upstream participant in both pathways. These results identify FUS as a new factor in the immediate response to DSBs that functions downstream of PAR polymerase to preserve genomic integrity.
Keywords: ATM; DNA Damage Response; DNA Repair; Fused in Sarcoma; Homologous Recombination; PARP; RNA-binding Proteins; Radiation Biology.
Figures
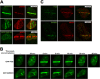
FIGURE 1.
FUS is recruited to sites of DNA damage. A, detection of GFP-FUS and GFP-TDP-43 at LIDD. U-2 OS cells expressing the indicated GFP-tagged protein were laser-microirradiated, fixed within 10 min, and processed for indirect immunofluorescence with the indicated antibodies. B, time course of GFP-FUS and GFP-EWSR1 occupancy at DNA damage sites. Cells expressing the indicated GFP-tagged protein were laser-microirradiated and monitored by live cell imaging. Shown are representative images from at least two independent experiments. C, detection of endogenous FUS and EWSR1 at LIDD. ∼50 cells were laser-microirradiated within 15 min. Cells were extracted prior to fixation and processed with the indicated antibodies.
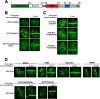
FIGURE 2.
PARP-dependent recruitment of FUS to sites of DNA damage. A, domain architecture of FUS is shown. NLS, nuclear localization signal. Mutations in the RRM or ZNF of FUS do not disrupt targeting to DNA damage sites. B, U-2 OS cells expressing the indicated GFP-FUS constructs were laser-microirradiated and monitored by live cell imaging. C, ALS-associated mutations in FUS do not abrogate localization to LIDD. U-2 OS cells expressing GFP-Myc-FUS(WT), R521G, or R524S were laser-microirradiated and monitored by live cell imaging. D, FUS localization to sites of DNA damage is PARP-dependent. U-2 OS cells expressing GFP-FUS(WT), S42A, or FUS4A were laser-microirradiated, and recruitment was monitored by live cell imaging. Where indicated, cells expressing GFP-FUS(WT) were pretreated with ATM inhibitor, DNA-PK inhibitor, or PARP inhibitor for 1 h prior to damage. Shown are representative images from at least two independent experiments.
FIGURE 3.
FUS interacts with PAR. A, HA-FUS protein was immunopurified (IP) from U-2 OS cells and incubated with 25 n
m
PAR for 1 h. The interaction was examined by dot-blotting (WB) with anti-PAR antibody. B, endogenous FUS was immunopurified from U-2 OS cells expressing shRNA against either a nontargeting (NT) control sequence or the 3′-UTR of FUS (FUS #2). PAR binding was performed as in A. Shown are representative blots from at least two independent experiments. EV, empty vector.
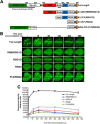
FIGURE 4.
The RGG2 domain is sufficient to accumulate at LIDD. A, FUS truncation and internal deletion mutants used in this study are depicted. B, U-2 OS cells expressing the indicated GFP-FUS constructs were laser-microirradiated and monitored by live cell imaging. C, FUS accumulation at sites of DNA damage (purple boxes) at the indicated time points was quantified using ImageJ software. FUS accumulation was normalized by subtracting the fluorescence intensity prior to microirradiation for each cell analyzed. A minimum of five cells were analyzed from two independent experiments. Error bars, S.E. from two independent experiments.
FIGURE 5.
The minimal FUS RGG2 domain binds PAR. A, U-2 OS cells expressing GFP-FUS(RGG2) were mock- or pretreated with PARP inhibitor for 1 h prior to laser microirradiation. Recruitment was monitored by live cell imaging. Shown are representative images from at least two independent experiments. B, GFP alone, GFP-FUS, or GFP-FUS(RGG2) proteins were immunopurified (IP) from U-2 OS cells and incubated with 25 n
m
PAR for 1 h. The interaction was examined by dot-blotting (WB) with anti-PAR antibody. Shown are representative blots from at least two independent experiments.
FIGURE 6.
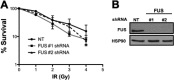
FUS depletion confers modest radiosensitivity in human cells. A, clonogenic survival in response to IR of U-2 OS cells expressing shRNA against a NT control sequence, FUS CDS (FUS #1), or FUS 3′-UTR (FUS #2). Error bars, S.E. from two independent experiments. B, Western blot showing FUS expression in shRNA cell lines.
FIGURE 7.
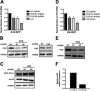
FUS is required for efficient DSB repair by NHEJ and HR. A and D, HEK-293 EJ5-GFP cells (A) or HEK-293 DR-GFP cells (D) were infected with lentivirus to deliver NT, FUS, Lig4, or CtIP shRNA and incubated for 48 h at which time, cells were transfected with plasmids encoding I-SceI and mCherry and incubated for an additional 72 h. A–E, cells were then harvested and analyzed by flow cytometry (A and D) or Western blotting (B, C, and E). F, CtIP knockdown was by evaluated quantitative PCR. Shown in A and D are repair frequencies relative to the NT shRNA control from at least three independent experiments, except for the Lig4 control, which represents technical replicates. Asterisks denote a statistical difference of p < 0.01 compared with the NT shRNA control.
Similar articles
- FUS RRM regulates poly(ADP-ribose) levels after transcriptional arrest and PARP-1 activation on DNA damage.
Mamontova EM, Clément MJ, Sukhanova MV, Joshi V, Bouhss A, Rengifo-Gonzalez JC, Desforges B, Hamon L, Lavrik OI, Pastré D. Mamontova EM, et al. Cell Rep. 2023 Oct 31;42(10):113199. doi: 10.1016/j.celrep.2023.113199. Epub 2023 Oct 5. Cell Rep. 2023. PMID: 37804508 - PARP activation regulates the RNA-binding protein NONO in the DNA damage response to DNA double-strand breaks.
Krietsch J, Caron MC, Gagné JP, Ethier C, Vignard J, Vincent M, Rouleau M, Hendzel MJ, Poirier GG, Masson JY. Krietsch J, et al. Nucleic Acids Res. 2012 Nov 1;40(20):10287-301. doi: 10.1093/nar/gks798. Epub 2012 Aug 31. Nucleic Acids Res. 2012. PMID: 22941645 Free PMC article. - PARP-1 dependent recruitment of the amyotrophic lateral sclerosis-associated protein FUS/TLS to sites of oxidative DNA damage.
Rulten SL, Rotheray A, Green RL, Grundy GJ, Moore DA, Gómez-Herreros F, Hafezparast M, Caldecott KW. Rulten SL, et al. Nucleic Acids Res. 2014 Jan;42(1):307-14. doi: 10.1093/nar/gkt835. Epub 2013 Sep 18. Nucleic Acids Res. 2014. PMID: 24049082 Free PMC article. - Roles of RNA-Binding Proteins in DNA Damage Response.
Kai M. Kai M. Int J Mol Sci. 2016 Feb 27;17(3):310. doi: 10.3390/ijms17030310. Int J Mol Sci. 2016. PMID: 26927092 Free PMC article. Review. - Fused in Sarcoma (FUS) in DNA Repair: Tango with Poly(ADP-ribose) Polymerase 1 and Compartmentalisation of Damaged DNA.
Sukhanova MV, Singatulina AS, Pastré D, Lavrik OI. Sukhanova MV, et al. Int J Mol Sci. 2020 Sep 24;21(19):7020. doi: 10.3390/ijms21197020. Int J Mol Sci. 2020. PMID: 32987654 Free PMC article. Review.
Cited by
- Proteomic analysis of FUS interacting proteins provides insights into FUS function and its role in ALS.
Kamelgarn M, Chen J, Kuang L, Arenas A, Zhai J, Zhu H, Gal J. Kamelgarn M, et al. Biochim Biophys Acta. 2016 Oct;1862(10):2004-14. doi: 10.1016/j.bbadis.2016.07.015. Epub 2016 Jul 25. Biochim Biophys Acta. 2016. PMID: 27460707 Free PMC article. - Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degenerations: Similarities in Genetic Background.
Parobkova E, Matej R. Parobkova E, et al. Diagnostics (Basel). 2021 Mar 13;11(3):509. doi: 10.3390/diagnostics11030509. Diagnostics (Basel). 2021. PMID: 33805659 Free PMC article. Review. - Comparative interactomics analysis of different ALS-associated proteins identifies converging molecular pathways.
Blokhuis AM, Koppers M, Groen EJN, van den Heuvel DMA, Dini Modigliani S, Anink JJ, Fumoto K, van Diggelen F, Snelting A, Sodaar P, Verheijen BM, Demmers JAA, Veldink JH, Aronica E, Bozzoni I, den Hertog J, van den Berg LH, Pasterkamp RJ. Blokhuis AM, et al. Acta Neuropathol. 2016 Aug;132(2):175-196. doi: 10.1007/s00401-016-1575-8. Epub 2016 May 10. Acta Neuropathol. 2016. PMID: 27164932 Free PMC article. - The Role of VCP Mutations in the Spectrum of Amyotrophic Lateral Sclerosis-Frontotemporal Dementia.
Scarian E, Fiamingo G, Diamanti L, Palmieri I, Gagliardi S, Pansarasa O. Scarian E, et al. Front Neurol. 2022 Feb 22;13:841394. doi: 10.3389/fneur.2022.841394. eCollection 2022. Front Neurol. 2022. PMID: 35273561 Free PMC article. Review. - Physiological, Pathological, and Targetable Membraneless Organelles in Neurons.
Ryan VH, Fawzi NL. Ryan VH, et al. Trends Neurosci. 2019 Oct;42(10):693-708. doi: 10.1016/j.tins.2019.08.005. Epub 2019 Sep 5. Trends Neurosci. 2019. PMID: 31493925 Free PMC article. Review.
References
- Harper J. W., Elledge S. J. (2007) The DNA damage response: ten years after. Mol. Cell 28, 739–745 - PubMed
- Matsuoka S., Ballif B. A., Smogorzewska A., McDonald E. R., 3rd, Hurov K. E., Luo J., Bakalarski C. E., Zhao Z., Solimini N., Lerenthal Y., Shiloh Y., Gygi S. P., Elledge S. J. (2007) ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 - PubMed
- Rogakou E. P., Pilch D. R., Orr A. H., Ivanova V. S., Bonner W. M. (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 - PubMed
- Lee J. H., Paull T. T. (2007) Activation and regulation of ATM kinase activity in response to DNA double-strand breaks. Oncogene 26, 7741–7748 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous