Transfection efficiency of chitosan and thiolated chitosan in retinal pigment epithelium cells: A comparative study - PubMed (original) (raw)
Transfection efficiency of chitosan and thiolated chitosan in retinal pigment epithelium cells: A comparative study
Ana V Oliveira et al. J Pharm Bioallied Sci. 2013 Apr.
Abstract
Objective: Gene therapy relies on efficient vector for a therapeutic effect. Efficient non-viral vectors are sought as an alternative to viral vectors. Chitosan, a cationic polymer, has been studied for its gene delivery potential. In this work, disulfide bond containing groups were covalently added to chitosan to improve the transfection efficiency. These bonds can be cleaved by cytoplasmic glutathione, thus, releasing the DNA load more efficiently.
Materials and methods: Chitosan and thiolated chitosan nanoparticles (NPs) were prepared in order to obtain a NH3(+):PO(4) (-) ratio of 5:1 and characterized for plasmid DNA complexation and release efficiency. Cytotoxicity and gene delivery studies were carried out on retinal pigment epithelial cells.
Results: In this work, we show that chitosan was effectively modified to incorporate a disulfide bond. The transfection efficiency of chitosan and thiolated chitosan varied according to the cell line used, however, thiolation did not seem to significantly improve transfection efficiency.
Conclusion: The apparent lack of improvement in transfection efficiency of the thiolated chitosan NPs is most likely due to its size increase and charge inversion relatively to chitosan. Therefore, for retinal cells, thiolated chitosan does not seem to constitute an efficient strategy for gene delivery.
Keywords: Chitosan; gene therapy; non-viral vectors; thiolation; transfection efficiency.
Conflict of interest statement
Conflict of Interest: None declared.
Figures
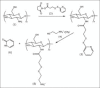
Figure 1
Reaction mechanism of N-succinimidyl-3-(2-pyridyldithio)- propionate (2) with chitosan, (1) resulting in chitosan-3-(2-pyridyldithio) propionyl (CS-PDP) (3) and hydroxysuccinimide. CS-PDP reacts with mercaptoetilamine salt, (4) producing chitosan-3-(2-Aminoethyldithio) propionyl-chitosan-3-(2-aminoethyldithio) propionyl (5) and pyridine- 2-thione (6)
Figure 2
Both chitosan (CS) and chitosan-3-(2-aminoethyldithio) propionyl (CS-(AEDTP)) nanoparticles (NPs) effectively protect DNA from DNAse degradation, as analyzed in a 1% agarose gel electrophoresis, with DNA visualized by GreenSafe Premium. Lanes: (a) DNA marker, (b) Plasmid DNA (pDNA), (c) pDNA + DNAse I, (d) CS-pDNA NPs, (e) CS-pDNA NPs + DNAse I, (f) CS-(AEDTP)- pDNA NPs, (g) CS-(AEDTP)-pDNA + DNAse I
Figure 3
DNA retention by chitosan-3-(2-aminoethyldithio) propionyl (CS-(AEDTP)) nanoparticles (NPs) after 24h of incubation with: A) Increasing concentrations of dithiothreitol; Lanes: (a) DNA marker, (b) Plasmid DNA (pDNA), (c) CS-(AEDTP) NPs, (d) to i) range from 10 mM to 100 mM according to the image. B) 0.4 M gluthatione reduced-form; Lanes: (a) DNA marker, (b) pDNA, (c) CS-(AEDTP) NPs, (d) CS-(AEDTP) NPs + NADPH + phosphate buffer, (e) CS-(AEDTP) NPs + glutathione reductase + NADPH + phosphate buffer, (f) CS-(AEDTP) NPs + PB + NADPH + phosphate buffer, (g) CS-(AEDTP) NPs + PB + GR+ NADPH + phosphate buffer. This was analyzed in a 1% agarose gel electrophoresis, with DNA visualized by GreenSafe Premium
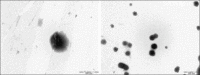
Figure 4
Transmission electron microscopy microphotographs of chitosan (CS) nanoparticles and DNA loaded nanoparticles (CSDNA 5:1)
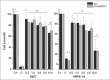
Figure 5
Cell survival (%) as a function of CS and chitosan-3- (2-aminoethyldithio) propionyl nanoparticles amount (μg of polymer); D407 and ARPE-19 cells were incubated for 72 h with the various concentrations of nanoparticles; (C+) untreated cells, (C−) cells treated with latex extract. Vertical bars = S.D. The number of *indicates significantly different sets of data
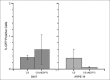
Figure 6
Transfection efficiency represented as GFP positive cell percentage as a function of polymer
Similar articles
- Chitosan-thioglycolic acid conjugate: an alternative carrier for oral nonviral gene delivery?
Martien R, Loretz B, Thaler M, Majzoob S, Bernkop-Schnürch A. Martien R, et al. J Biomed Mater Res A. 2007 Jul;82(1):1-9. doi: 10.1002/jbm.a.31135. J Biomed Mater Res A. 2007. PMID: 17265441 - Preparation and evaluation of chitosan-DNA-FAP-B nanoparticles as a novel non-viral vector for gene delivery to the lung epithelial cells.
Mohammadi Z, Abolhassani M, Dorkoosh FA, Hosseinkhani S, Gilani K, Amini T, Najafabadi AR, Tehrani MR. Mohammadi Z, et al. Int J Pharm. 2011 May 16;409(1-2):307-13. doi: 10.1016/j.ijpharm.2011.02.043. Epub 2011 Feb 26. Int J Pharm. 2011. PMID: 21356293 - Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems.
Chen J, Tian B, Yin X, Zhang Y, Hu D, Hu Z, Liu M, Pan Y, Zhao J, Li H, Hou C, Wang J, Zhang Y. Chen J, et al. J Biotechnol. 2007 Jun 15;130(2):107-13. doi: 10.1016/j.jbiotec.2007.02.007. Epub 2007 Feb 17. J Biotechnol. 2007. PMID: 17467097 - Chitosan-DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy.
Mansouri S, Lavigne P, Corsi K, Benderdour M, Beaumont E, Fernandes JC. Mansouri S, et al. Eur J Pharm Biopharm. 2004 Jan;57(1):1-8. doi: 10.1016/s0939-6411(03)00155-3. Eur J Pharm Biopharm. 2004. PMID: 14729076 Review. - [Progress in research of chitosan as a non-viral gene delivery vector].
Su HS, Wang YF. Su HS, et al. Yi Chuan. 2006 Oct;28(10):1321-4. doi: 10.1360/yc-006-1321. Yi Chuan. 2006. PMID: 17035195 Review. Chinese.
Cited by
- Thiolated Nanoparticles for Biomedical Applications: Mimicking the Workhorses of Our Body.
Hock N, Racaniello GF, Aspinall S, Denora N, Khutoryanskiy VV, Bernkop-Schnürch A. Hock N, et al. Adv Sci (Weinh). 2022 Jan;9(1):e2102451. doi: 10.1002/advs.202102451. Epub 2021 Nov 12. Adv Sci (Weinh). 2022. PMID: 34773391 Free PMC article. Review. - TAT-LHRH conjugated low molecular weight chitosan as a gene carrier specific for hepatocellular carcinoma cells.
Liu L, Dong X, Zhu D, Song L, Zhang H, Leng XG. Liu L, et al. Int J Nanomedicine. 2014 Jun 10;9:2879-89. doi: 10.2147/IJN.S61392. eCollection 2014. Int J Nanomedicine. 2014. PMID: 24959076 Free PMC article. - Chitosan combined with molecular beacon for mir-155 detection and imaging in lung cancer.
Zhu HZ, An JH, Yao Q, Han J, Li XT, Jiang FL, Chen GP, Peng LN, Li YS, Sun JG, Chen ZT. Zhu HZ, et al. Molecules. 2014 Sep 16;19(9):14710-22. doi: 10.3390/molecules190914710. Molecules. 2014. PMID: 25230125 Free PMC article. - Cationic polyene phospholipids as DNA carriers for ocular gene therapy.
Machado S, Calado S, Bitoque D, Oliveira AV, Øpstad CL, Zeeshan M, Sliwka HR, Partali V, Pungente MD, Silva GA. Machado S, et al. Biomed Res Int. 2014;2014:703253. doi: 10.1155/2014/703253. Epub 2014 Jul 24. Biomed Res Int. 2014. PMID: 25147812 Free PMC article. - Tailoring the properties of chitosan by grafting with 2-mercaptobenzoic acid to improve mucoadhesion: in silico studies, synthesis and characterization.
Marwaha TK, Madgulkar A, Bhalekar M, Asgaonkar K, Gachche R, Shewale P. Marwaha TK, et al. Prog Biomater. 2022 Dec;11(4):397-408. doi: 10.1007/s40204-022-00201-x. Epub 2022 Oct 7. Prog Biomater. 2022. PMID: 36205916 Free PMC article.
References
- Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–9. - PubMed
- Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, Gross F, Yvon E, Nusbaum P, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–72. - PubMed
- Robbins PD, Tahara H, Ghivizzani SC. Viral vectors for gene therapy. Trends Biotechnol. 1998;16:35–40. - PubMed
- Gôrecki DC. “Dressed-up” naked plasmids: Emerging vectors for non-viral gene therapy. Discov Med. 2006;6:191–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources